Abstract
Introduction
Topical rapamycin has been established as an effective and safe therapy for facial angiofibromas in tuberous sclerosis. Different formulations have been tested for this skin disease, most using an ointment as a vehicle.
Purpose
To improve the classical formulation of topical rapamycin and to determine the validity period of the proposed options based on chemical, physical, and microbiological stability studies.
Methods
Four different 0.4% rapamycin formulations were prepared (ointment, emulsion, gel, and liposomes). The stability studies for each formulation over 56 days were as follows: (1) chemical: extraction with different solvents and high-performance liquid chromatography assay; (2) physical: pH, uniformity, extensibility, absence of crystals, absence of phase separation, and only for liposomal formulation, particle size, zeta potential, and encapsulation efficiency were determined; and (3) microbiological: culture samples in blood-agar media.
Results
Only liposomes were chemically, physically, and microbiologically stable after 8 weeks. Ointment, emulsion, and gel formulations lost their chemical or physical stability before 56 days.
Conclusions
The authors describe four new formulations to improve the previous treatment for facial angiofibromas in tuberous sclerosis. The liposome-based formulation was the most appropriate according to chemical, physical, and microbiological stability studies. However, it would be necessary to carry out clinical studies to ensure the effectiveness and safety of this formulation and also guarantee an improvement in the quality of life of patients.
Similar content being viewed by others
Introduction
Tuberous sclerosis (TS) is an autosomal dominant rare disease, characterized by the multisystem formation of benign non-invasive tumors, called hamartomas and distributed in tissues and organs such as the brain, kidneys, lungs, heart, eyes, and skin [1]. The most prevalent skin condition in TS is facial angiofibromas (FA), which occur due to an alteration of the mTOR (mammalian target of rapamycin) pathway. These facial injuries imply an aesthetic and psychological problem [2, 3], and sometimes, its treatment is not prioritized due to the existence of more serious complications associated with this rare disease.
Multiple treatments have been established for these patients. Physical treatments like radiofrequency, electrocoagulation, cryotherapy, dermabrasion, and laser therapy [4] are invasive and painful and require anesthesia. The interest of mTOR inhibitors focuses pharmacological treatment on everolimus and sirolimus, and recent publications place topical rapamycin (sirolimus) as the most appropriate alternative [5].
We found multiple studies with topical rapamycin in the literature, including four randomized clinical trials [6,7,8,9], and all of them provide favorable data on effectiveness and safety. However, high variability between studies in terms of concentration, vehicle, posology, duration of treatment, and effectiveness assessment forces us to study several formulations to choose the most appropriate.
Several authors have studied different formulations other than the classic formulation (an ointment based on petrolatum) with the aim of achieving a better appearance and consistency of the formulation in order to improve patient compliance with treatment.
Bouguéon et al. [10] studied the physico-chemical stability of a cream formulation with a commercialized excipient called Excipial Hydrocrème®. Additionally, they used diethylene glycol monoethyl ether P (Transcutol®) as a vehicle and demonstrated that rapamycin was ten times more soluble in Transcutol® (fully soluble, 20.2 mg/mL) compared to liquid paraffin (< 2 mg/mL). Le Guyader et al. [11] conducted a physico-chemical and microbiological stability study on a gel-type formulation. These authors also recommend the use of solubilizing agents such as transcutol in combination with gel-type formulations, since they have demonstrated improved permeation, penetration, and release. Ghanbarzadeh et al. formulated rapamycin integrated into a liposomal solution, explaining the methodology in development of rapamycin liposomes, and additionally studied their chemical stability using a validated high-performance liquid chromatography (HPLC) method [12, 13].
Among all the concentrations and dosages reported in the literature, 0.4% concentration, three times per week, is interesting to avoid exposure to the drug every day and thus minimize possible cutaneous adverse events without compromising effectiveness [14, 15].
In addition, the current legal regulations in Spain on magistral formulas establish that the validity period of non-typified magistral formulas corresponds to the full duration of the treatment [16]. Secondly, the Guide to Good Practice of Preparation of Medications in Hospital Pharmacy Services in Spain establishes a validity period of 30 days for ointments and creams, which can be increased if demonstrated with stability studies [17].
The purpose of this study is to improve the classic formulation of topical rapamycin for FA in TS and determine the validity period of the proposed formulations based on chemical, physical, and microbiological stability.
Methods
Materials and Reagents
Rapamycin powder (an active substance) was provided by Acofarma (Madrid, Spain). The rest of the excipients used in the preparation of the formulations were supplied by Guinama (Valencia, Spain). All reagents and solvents were provided by Scharlab (Valencia, Spain).
Formulation and Preparation
Galenic optimization of the topical formulation of rapamycin at 0.4% based on its excipients was performed. The most widely used classical formulation in the literature is petrolatum as a vehicle. However, other options were formulated based on previous studies.
Four different formulations were developed in the biological safety cabinet, all of them with a rapamycin concentration of 0.4% and with differences in their excipients. The resulting formulations were ointment, emulsion, gel, and liposomes (Table 1). Each formulation was packaged in a bottle protected from ambient light and stored in a cold room at 5 °C ± 3 °C.
Chemical Stability
Equipment
An Agilent Technologies 1100 HPLC system (Agilent Technologies Inc., Waldbronn, Karlsruhe, Germany) with a quaternary pump, micro-vacuum degasser, autosampler, thermostatted column compartment, diode array detector, and Agilent Technologies ChemStation for LC 3D Software was used for the analysis.
Chromatographic Conditions
To determine the validity period of each formulation according to its chemical stability, an HPLC method was developed and validated. A C18 Kromasil column (150 mm × 4.6 mm, 5 μm) was used. The mobile phase consisted of a mixture of acetonitrile and water (75/25 v/v). The flow rate was 1 mL/min. The sample injection volume was 10 μL and sample temperature was 25 °C ± 3 °C. The column temperature was 50 °C and the analysis time was 15 min. Rapamycin detection was processed at 280 nm.
Method Validation
The analytical methods were validated according to ICHQ2R1 (International Consensus on Harmonization) [18]. Two calibration curves were developed: one using a mixture of acetonitrile, hexane, and water for injection (WFI) as a diluent (for the chemical stability of ointment, emulsion, and gel formulations) and the other using methanol (for the chemical stability of liposomes). The interday and intraday precision and accuracy of the methods were established using six concentration levels (0.025, 0.05, 0.075, 0.1, 0.125, and 0.15 mg/mL) in duplicate on three different days. The least squares method was used to evaluate linearity, calculating a regression line between concentrations and peak areas of the chromatogram.
Rapamycin Extraction
For the extraction of rapamycin from ointment, emulsion, and gel formulations, 0.1 g of cream was introduced in a glass tube with 4 mL of a mixture of acetonitrile, hexane, and WFI and kept in the vortex for 10 s. The samples were then centrifuged at 3500 rpm for 10 min. The resulting supernatant was removed, and 1 mL was aliquoted for HPLC analysis. For the extraction of rapamycin from liposomes, 0.100 g of cream was introduced in a glass tube with 4 mL of methanol. The samples were kept in the vortex for 10 s, 50 μL was aliquoted, and after 1/20 dilution with methanol, they were analyzed with the HPLC method.
Rapamycin Degradation
Rapamycin quantity per weighed formulation quantity (Q, mg/g) and percentage content of remaining rapamycin (%RC) in each formulation were determined by triplicate at times (t) = 0, 2, 7, 14, 21, 28, 42, and 56 days. T90 was established when %CR was ≤ 90%.
Physical Stability
All pertinent procedures to establish the physical stability of the formulations were carried out following the specifications of the National Drug Formulary [19] and the Guide to Good Practice in the Preparation of Medications in Hospital Pharmacy Services [17].
pH, uniformity, extensibility, absence of crystals, and absence of phase separations were evaluated on a transparent surface according to 3 levels: level 1, the least favorable, and level 3, the most favorable, for 56 days for each formulation.
Liposome Characterization
Only for liposomes mean particle size, zeta potential, and encapsulation efficiency (EE%) were determined by triplicate at t = 0 days. Mean particle size and zeta potential were determined using a particle size analyzer that uses the laser diffraction method (Malvern Instruments, Worcestershire, UK). The EE% was obtained via ultrafiltration, using Amicon® ultracentrifugal filters (Merck Millipore, Ireland) with a molecular weight cutoff of 10,000 Da. An aliquot of 500 μL of the liposomal formulation was added to the sample reservoir and centrifuged for 15 min at 14,000 g. Then, 50 μL was aliquoted in duplicate and, after 1/20 dilution with methanol, was analyzed with HPLC method to determine the concentration of free drug in the filtrate. The following equations were used for the calculations [13, 20]:
where Wt and Wf represent the total amount of the drug and the free amount of the drug, respectively.
Microbiological Stability
Culture samples in blood-agar media of each formulation were incubated at 37 °C in duplicate at t = 28 and 56 days, according to the microbiological control instructions of the National Drug Formulary.
Results
Chemical Stability
Method Validation
The calibration curve for the chemical stability of ointment, emulsion, and gel formulations demonstrated linearity with a coefficient of determination (r2) of 0.9998. The limits of detection (LD) and quantification (LQ) were 0.003 and 0.009 mg/mL, respectively. Linearity was also determined for the chemical stability of the liposomal formulation, with a coefficient of determination (r2) of 0.9958, LD = 0.011 mg/mL, and LQ = 0.038 mg/mL.
Rapamycin Degradation
Table 2 shows the rapamycin quantity per weighed formulation quantity (Q, mg/g) and the percentage content of remaining rapamycin (%RC) in each formulation at times (t) = 0, 7, 14, 21, 28, 42, and 56 days. Figure 1 shows ointment, emulsion, gel, and liposome chromatograms.
T90 was reached during the sampling period for the ointment, emulsion, and gel formulations: T90 = 56, 14, and 21 days, respectively. Only liposomes were stable for 56 days.
Physical Stability
Table 3 exposes the results of the physical stability parameters for the four formulations. The ointment formulation obtained a level 1 score in extensibility property due to its high petrolatum content, similar to the classical formulation. It should be noted that the emulsion formulation resulted in a pre-separation of phases at 14 days and a definitive breakdown of the emulsion at 21 days; therefore, it was assigned a level 1 score in the absence of phase separations property. The score of level 1 in the absence of crystal property for the gel formulation was awarded by the presence of white particles.
Liposome Characterization
The mean particle size of prepared liposomes determined a good quality result of the analysis with a polydispersity index value < 0.2, indicating a homogenous dispersion. The EE% turned out to be very favorable for the liposomal formulation, with a high load of rapamycin per liposome formed.
Microbiological Stability
Culture samples were negative in blood-agar media for each formulation at t = 28 and 56 days (Table 3).
Discussion
This study concludes that only one of the four proposed formulations, liposomes, maintains their chemical, physical, and microbiological stability throughout all the sampling time, awarding them a validity period of 56 days.
For the ointment and gel formulations, the chemical stability was the determining factor for not reaching the validity period of 56 days, with T90 = 56 and 21 days, respectively. In the case of the emulsion formulation, the physical stability was very obviously compromised after pre-separation of phases at 14 days and a definitive breaking up of the emulsion at 21 days. All formulations were microbiologically stable throughout the sampling period. Considering all stability studies, the validity period for each formulation was as follows: ointment = 42 days, emulsion = 7 days, gel = 14 days, and liposomes = 56 days.
Rapamycin is a highly apolar, active substance. This stability study confirms that rapamycin is more comfortable among lipophilic excipients, such as petrolatum and liposomes. In hydrophilic vehicles, it tends to precipitate and lose its physical and chemical stability, as in the case of emulsion and gel formulations.
Regarding excipients, transcutol allows a greater solubility of rapamycin in the vehicle [10], thus avoiding possible precipitations of the active principle and constituting a significant improvement in the formulation.
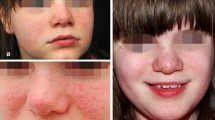
The liposomal formulation has certain advantages over the ointment formulation: it has a better appearance, is more comfortable, and has better extensibility (Photograph 1). Historically, some patients complained that classic ointment was difficult to apply [21] and the enhanced organoleptic properties of the liposomal formulation would help increase compliance with treatment and patient satisfaction with topical therapy.
Additionally, liposomes have been studied as carriers of molecules for immunosuppressive therapies and chemotherapy [22, 23]. Liposome-encapsulated rapamycin has been shown to have the ability to reach the drug’s site of action more quickly, with antiproliferative properties on T cells [23, 24].
Therefore, liposomes exert their influence on the stratum corneum of the skin, improving the penetration of the active substance and providing an increased bioavailability of rapamycin [20]. This property makes liposomes particularly effective for cosmetic and pharmaceutical use.
In these therapies, the role of the hospital pharmacists is key in the preparation of the formulations, since rapamycin was added to the NIOSH list as a hazardous drug in 2014 [25], which results in greater control in the development of topical formulations [26]. In addition, pharmaceutical care has special relevance in the processes of dispensing, administration, and monitoring, promoting the supervision and control of these patients.
After galenic improvement and confirmation of stability in liposomes, clinical studies are needed to ensure effectiveness, safety, and high patient satisfaction.
Limitations
The authors are aware that there is significant variability in chemical stability results for all formulations. This is due to the fact that during the formulation process, the correct homogenization of the excipients is very difficult, and the extractability of solvents depends on the formulation and its excipients. In any case, this article provides new stability data, allowing support in therapeutic decisions for other authors and clinicians.
References
Narayanan V. Tuberous sclerosis complex: genetics to pathogenesis. Pediatr Neurol. 2003;29(5):404–9.
Rumsey N, Clarke A, White P. Exploring the psychosocial concerns of outpatients with disfiguring conditions. J Wound Care. 2003;12(7):247–52.
Zweegers J, van der Vleuten CJ. The psychosocial impact of an infantile haemangioma on children and their parents. Arch Dis Child. 2012;97(10):922–6.
Biondo G, Greco S, Mavilia L, Mercuri SR. Treatment of nodular facial angiofibromas in tuberous sclerosis, using ultrapulse carbon dioxide laser. Clin Exp Dermatol. 2014;39(6):738–40.
Balestri R, Neri I, Patrizi A, Angileri L, Ricci L, Magnano M. Analysis of current data on the use of topical rapamycin in the treatment of facial angiofibromas in tuberous sclerosis complex. J Eur Acad Dermatol Venereol. 2015;29(1):14–20.
Koenig MK, Hebert AA, Roberson J, Samuels J, Slopis J, Woerner A, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R D. 2012;12(3):121–6.
Wataya-Kaneda M, Nakamura A, Tanaka M, Hayashi M, Matsumoto S, Yamamoto K, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex : a randomized clinical trial. JAMA Dermatol. 2017;153(1):39–48.
Koenig MK, Bell CS, Hebert AA, Roberson J, Samuels JA, Slopis JM, et al. Efficacy and safety of topical rapamycin in patients with facial angiofibromas secondary to tuberous sclerosis complex: the TREATMENT randomized clinical trial. JAMA Dermatol. 2018;154(7):773–80.
Chen PL, Hong JB, Shen LJ, Chen YT, Wang SJ, Liao YH. The efficacy and safety of topical rapamycin-calcitriol for facial angiofibromas in patients with tuberous sclerosis complex: a prospective, double-blind, randomized clinical trial. Br J Dermatol. 2020;183(4):655–63.
Bougueon G, Lagarce F, Martin L, Pailhories H, Bastiat G, Vrignaud S. Formulation and characterization of a 0.1% rapamycin cream for the treatment of tuberous sclerosis complex-related angiofibromas. Int J Pharm. 2016;509(1–2):279–84.
Le Guyader G, Vieillard V, Andrieux K, Rollo M, Thirion O, Wolkenstein P, et al. Long-term stability of 0.1% rapamycin hydrophilic gel in the treatment of facial angiofibromas. Eur J Hosp Pharm. 2020;27(e1):e48–52.
Ghanbarzadeh S, Valizadeh H, Zakeri-Milani P. Application of response surface methodology in development of sirolimus liposomes prepared by thin film hydration technique. Bioimpacts. 2013;3(2):75–81.
Ghanbarzadeh S, Valizadeh H, Zakeri-Milani P. The effects of lyophilization on the physico-chemical stability of sirolimus liposomes. Adv Pharm Bull. 2013;3(1):25–9.
Salido R, Garnacho-Saucedo G, Cuevas-Asencio I, Ruano J, Galan-Gutierrez M, Velez A, et al. Sustained clinical effectiveness and favorable safety profile of topical sirolimus for tuberous sclerosis - associated facial angiofibroma. J Eur Acad Dermatol Venereol. 2012;26(10):1315–8.
Tiedemann Svendsen M, Bygum A, Hansen LK, Broesby-Olsen S. Facial angiofibromas associated to tuberous sclerosis treated with topical sirolimus. Ugeskr Laeger. 2013;175(43):2569–70.
Ministry of Health, Consumption and Social Welfare. Madrid, Spain. Royal Decree 175/2001, of 23 February, which approves the standards of correct preparation and quality control of magistral formulas and officinal preparations. 2001. Available at: https://www.boe.es/buscar/pdf/2001/BOE-A-2001-5185-consolidado.pdf. Accessed Sep 2023.
Ministry of Health, Consumption and Social Welfare. Madrid, Spain. Guide to good practice of preparation of medications in hospital pharmacy services. June, 2014. Available at: https://www.mscbs.gob.es/profesionales/farmacia/pdf/GuiaBPP3.pdf. Accessed Sep 2023.
International Conference on Harmonisation. Validation of analytical procedures: text and methodology. 2015: 6–12.
Ministry of Health, Consumption and Social Welfare, by mandate of Law 29/2006, of July 26, on guarantee and rational use of medicines and health products. National Drug Formulary. Madrid, Spain. 2019.; Available at: https://www.boe.es/biblioteca_juridica/abrir_pdf.php?id=PUB-NT-2019-112. Accessed Sep 2023.
Yoon HY, Chang IH, Goo YT, Kim CH, Kang TH, Kim SY, et al. Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel. Int J Nanomedicine. 2019;5(14):6249–68.
Park J, Yun SK, Cho YS, Song KH, Kim HU. Treatment of angiofibromas in tuberous sclerosis complex: the effect of topical rapamycin and concomitant laser therapy. Dermatology. 2014;228(1):37–41.
Onyesom I, Lamprou DA, Sygellou L, Owusu-Ware SK, Antonijevic M, Chowdhry BZ, et al. Sirolimus encapsulated liposomes for cancer therapy: physicochemical and mechanical characterization of sirolimus distribution within liposome bilayers. Mol Pharm. 2013;10(11):4281–93.
Rouf MA, Vural I, Renoir JM, Hincal AA. Development and characterization of liposomal formulations for rapamycin delivery and investigation of their antiproliferative effect on MCF7 cells. J Liposome Res. 2009;19(4):322–31.
Valizadeh H, Ghanbarzadeh S, Zakeri-Milani P. Fusogenic liposomal formulation of sirolimus: improvement of drug anti-proliferative effect on human T-cells. Drug Dev Ind Pharm. 2015;41(9):1558–65.
Department of Health and Human Services. Centers for Disease Control and Prevention National Institute for Occupational Safety and Health. NIOSH list of antineoplastic and other hazardous drugs in healthcare settings, 2014. Available in: https://www.cdc.gov/niosh/docs/2014-138/pdfs/2014-138.pdf. 2014. Accessed Sep 2023.
Cortell Fuster C, Martínez Gómez MA, Cercós Lleti AC, Climente Martí M. Topical rapamycin in the treatment of facial angiofibromas in tuberous sclerosis: a systematic review based on evidence. J Dermatolog Treat. 2021;6:1–7.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Key Findings
• The results obtained allow a substantial improvement of the therapy in terms of galenic optimization and provide favorable stability data for the liposomal formulation developed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cortell-Fuster, C., Martínez-Gómez, M., Cercós-Lleti, A. et al. Optimization, Formulation, and Stability of Topical Rapamycin Used for Rare Tuberous Sclerosis Disease: from Ointment to Liposomes. J Pharm Innov 18, 2287–2293 (2023). https://doi.org/10.1007/s12247-023-09792-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09792-9