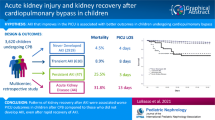
Abstract
Background
Cardiac surgery-associated acute kidney injury (CS-AKI) is common, but its impact on clinical outcomes is variable. Parsing AKI into sub-phenotype(s) and integrating pathologic positive cumulative fluid balance (CFB) may better inform prognosis. We sought to determine whether durational sub-phenotyping of CS-AKI with CFB strengthens association with outcomes among neonates undergoing the Norwood procedure.
Methods
Multicenter, retrospective cohort study from the Neonatal and Pediatric Heart and Renal Outcomes Network. Transient CS-AKI: present only on post-operative day (POD) 1 and/or 2; persistent CS-AKI: continued after POD 2. CFB was evaluated per day and peak CFB during the first 7 postoperative days. Primary and secondary outcomes were mortality, respiratory support-free and hospital-free days (at 28, 60 days, respectively). The primary predictor was persistent CS-AKI, defined by modified neonatal Kidney Disease: Improving Global Outcomes criteria.
Results
CS-AKI occurred in 59% (205/347) neonates: 36.6% (127/347) transient and 22.5% (78/347) persistent; CFB > 10% occurred in 18.7% (65/347). Patients with either persistent CS-AKI or peak CFB > 10% had higher mortality. Combined persistent CS-AKI with peak CFB > 10% (n = 21) associated with increased mortality (aOR: 7.8, 95% CI: 1.4, 45.5; p = 0.02), decreased respiratory support-free (predicted mean 12 vs. 19; p < 0.001) and hospital-free days (17 vs. 29; p = 0.048) compared to those with neither.
Conclusions
The combination of persistent CS-AKI and peak CFB > 10% after the Norwood procedure is associated with mortality and hospital resource utilization. Prospective studies targeting intra- and postoperative CS-AKI risk factors and reducing CFB have the potential to improve outcomes.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information


Similar content being viewed by others
Data availability
Requests for proposal concept papers and analyses can be made through the pediatric cardiac critical care consortium (PC4) at https://pc4quality.org/.
References
Blinder JJ, Asaro LA, Wypij D et al (2017) Acute kidney injury after pediatric cardiac surgery: a secondary analysis of the safe pediatric euglycemia after cardiac surgery trial. Pediatr Crit Care Med 18:638–646. https://doi.org/10.1097/PCC.0000000000001185
Blinder JJ, Goldstein SL, Lee V-V et al (2012) Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg 143:368–374. https://doi.org/10.1016/j.jtcvs.2011.06.021
Hirano D, Ito A, Yamada A et al (2017) Independent risk factors and 2-year outcomes of acute kidney injury after surgery for congenital heart disease. Am J Nephrol 46:204–209. https://doi.org/10.1159/000480358
Aydin SI, Seiden HS, Blaufox AD et al (2012) Acute kidney injury after surgery for congenital heart disease. Ann Thorac Surg 94:1589–1595. https://doi.org/10.1016/j.athoracsur.2012.06.050
Morgan CJ, Gill PJ, Lam S, Joffe AR (2013) Peri-operative interventions, but not inflammatory mediators, increase risk of acute kidney injury after cardiac surgery: a prospective cohort study. Intensive Care Med 39:934–941. https://doi.org/10.1007/s00134-013-2849-4
Park S-K, Hur M, Kim E et al (2016) Risk factors for acute kidney injury after congenital cardiac surgery in infants and children: a retrospective observational study. PLoS One 11:e0166328. https://doi.org/10.1371/journal.pone.0166328
Gil-Ruiz Gil-Esparza MA, Alcaraz Romero AJ, Romero Otero A et al (2014) Prognostic relevance of early AKI according to pRIFLE criteria in children undergoing cardiac surgery. Pediatr Nephrol 29:1265–1272. https://doi.org/10.1007/s00467-014-2757-z
Tanyildiz M, Ekim M, Kendirli T et al (2017) Acute kidney injury in congenital cardiac surgery: pediatric risk-injury-failure-loss-end-stage renal disease and acute kidney injury network. Pediatr Int 59:1252–1260. https://doi.org/10.1111/ped.13359
Watkins SC, Williamson K, Davidson M, Donahue BS (2014) Long-term mortality associated with acute kidney injury in children following congenital cardiac surgery. Paediatr Anaesth 24:919–926. https://doi.org/10.1111/pan.12419
Esch JJ, Salvin JM, Thiagarajan RR et al (2015) Acute kidney injury after Fontan completion: risk factors and outcomes. J Thorac Cardiovasc Surg 150:190–197. https://doi.org/10.1016/j.jtcvs.2015.04.011
Alten JA, Cooper DS, Blinder JJ et al (2021) Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med 49:e941–e951. https://doi.org/10.1097/CCM.0000000000005165
Goldstein SL, Akcan-Arikan A, Alobaidi R et al (2022) Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi consensus statement. JAMA Netw Open 5:e2229442. https://doi.org/10.1001/jamanetworkopen.2022.29442
Alobaidi R, Morgan C, Basu RK et al (2018) Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr 172:257–268. https://doi.org/10.1001/jamapediatrics.2017.4540
Arikan AA, Zappitelli M, Goldstein SL et al (2012) Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med 13:253–258. https://doi.org/10.1097/PCC.0b013e31822882a3
Flori HR, Church G, Liu KD et al (2011) Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract 2011:854142. https://doi.org/10.1155/2011/854142
Sutherland SM, Zappitelli M, Alexander SR et al (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55:316–325. https://doi.org/10.1053/j.ajkd.2009.10.048
Vaara ST, Bhatraju PK, Stanski NL et al (2022) Subphenotypes in acute kidney injury: a narrative review. Crit Care 26:251. https://doi.org/10.1186/s13054-022-04121-x
Hoste EA, Maitland K, Brudney CS et al (2014) Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth 113:740–747. https://doi.org/10.1093/bja/aeu300
Gist KM, Borasino S, SooHoo M et al (2021) Transient and persistent acute kidney injury phenotypes following the Norwood operation: a retrospective study. Cardiol Young 32:564–571. https://doi.org/10.1017/S1047951121002560
SooHoo MM, Patel SS, Jaggers J et al (2018) Acute kidney injury defined by fluid corrected creatinine in neonates after the Norwood procedure. World J Pediatr Congenit Heart Surg 9:513–521. https://doi.org/10.1177/2150135118775413
Gaies M, Cooper DS, Tabbutt S et al (2015) Collaborative quality improvement in the cardiac intensive care unit: development of the Paediatric Cardiac Critical Care Consortium (PC4). Cardiol Young 25:951–957. https://doi.org/10.1017/S1047951114001450
Gist KM, Blinder JJ, Bailly D et al (2019) Neonatal and paediatric heart and renal outcomes network: design of a multi-centre retrospective cohort study. Cardiol Young 29:511–518. https://doi.org/10.1017/S1047951119000210
Jetton JG, Boohaker LJ, Sethi SK et al (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194. https://doi.org/10.1016/S2352-4642(17)30069-X
Goldstein SL, Currier H, Graf Cd, Cosio CC, Brewer ED, Sachdeva R (2001) Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 107:1309–1312. https://doi.org/10.1542/peds.107.6.1309
Liu Q, Shepherd BE, Li C, Harrell FE (2017) Modeling continuous response variables using ordinal regression. Stat Med 36:4316–4335. https://doi.org/10.1002/sim.7433
Gaies MG, Gurney JG, Yen AH et al (2010) Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 11:234–238. https://doi.org/10.1097/PCC.0b013e3181b806fc
(2023) 4th International symposium on acute kidney injury in children. Pediatr Nephrol 38 (Suppl1):1–42. https://doi.org/10.1007/s00467-022-05843-4
LoBasso M, Schneider J, Sanchez-Pinto LN et al (2022) Acute kidney injury and kidney recovery after cardiopulmonary bypass in children. Pediatr Nephrol 37:659–665. https://doi.org/10.1007/s00467-021-05179-5
Mah KE, Hao S, Sutherland SM et al (2018) Fluid overload independent of acute kidney injury predicts poor outcomes in neonates following congenital heart surgery. Pediatr Nephrol 33:511–520. https://doi.org/10.1007/s00467-017-3818-x
Wilder NS, Yu S, Donohue JE et al (2016) Fluid overload is associated with late poor outcomes in neonates following cardiac surgery. Pediatr Crit Care Med 17:420–427. https://doi.org/10.1097/PCC.0000000000000715
Piggott KD, Soni M, Decampli WM et al (2015) Acute kidney injury and fluid overload in neonates following surgery for congenital heart disease. World J Pediatr Congenit Heart Surg 6:401–406. https://doi.org/10.1177/2150135115586814
Bailly DK, Alten JA, Gist KM et al (2022) Fluid accumulation after neonatal congenital cardiac operation: clinical implications and outcomes. Ann Thorac Surg 114:2288–2294. https://doi.org/10.1016/j.athoracsur.2021.12.078
Gist KM, Selewski DT, Brinton J et al (2020) Assessment of the independent and synergistic effects of fluid overload and acute kidney injury on outcomes of critically ill children. Pediatr Crit Care Med 21:170–177. https://doi.org/10.1097/PCC.0000000000002107
Pettit KA, Selewski DT, Askenazi DJ et al (2023) Synergistic association of fluid overload and acute kidney injury on outcomes in pediatric cardiac ECMO: a retrospective analysis of the KIDMO database. Pediatr Nephrol 38:1343–1353. https://doi.org/10.1007/s00467-022-05708-w
Liu KD, Altmann C, Smits G et al (2009) Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care 13:R104. https://doi.org/10.1186/cc7940
Selewski DT, Gist KM, Basu RK et al (2023) Impact of the magnitude and timing of fluid overload on outcomes in critically ill children: a report from the Multicenter International Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology (AWARE) Study. Crit Care Med 51:606–618. https://doi.org/10.1097/CCM.0000000000005791
Kaddourah A, Basu RK, Bagshaw SM et al (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20. https://doi.org/10.1056/NEJMoa1611391
Neumayr TM, Alten JA, Bailly DK et al (2023) Assessment of fluid balance after neonatal cardiac surgery: a description of intake/output vs. weight-based methods. Pediatr Nephrol 38:1355–1364. https://doi.org/10.1007/s00467-022-05697-w
Neonatal and Pediatric Heart and Renal Outcomes Network author list
The following individuals served as collaborators and investigators for the NEPHRON studies. They collaborated in protocol development and review and data analysis, and participated in drafting or review of the manuscript, and their names should be citable by PubMed.
Andrew Smith1, Katie L Brandewie2, Priya N Bhat3, John W Diddle4, Muhammed Ghbeis5, Kenneth E Mah6, Tara M Neumayr7, Tia T Raymond8, Parthak Prodhan9, Xiomara Garcia9, Shannon Ramer9, Mindy Albertson9, David S. Cooper2, Zahidee Rodriguez10, Mary Lukacs2, Michael Gaies2, Amanda Sammons2, Joan Sanchez de Toledo11, Yuliya A Domnina4, Lucas Saenz11, Tracy Baust11, Jane Kluck12, Joshua D Koch13, Jun Sasaki5, Aanish Raees2, Natasha S Afonso3, Erika R O’Neill3, Javier J Lasa3, Patrick A Phillips14, Kristal M Hock14, Santiago Borasino14, David Kwiatkowski6, Joshua Blinder6, Kevin Valentine15, Sachin Tadphale16, Jason R Buckley17, Shanelle Clarke10, Wenying Zhang18, Mohammed Absi16, David J Askenazi.14
1Johns Hopkins University, St. Petersburg, FL, USA.
2Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
3Texas Children’s Hospital, Houston, TX, USA.
4Children’s National Medical Center, Washington DC, USA.
5Boston Children’s Hospital, Boston, MA, USA.
6Stanford Children’s Hospital, Palo Alto, CA, USA.
7Washington University School of Medicine, St. Louis, MO, USA
8Medical City Children’s Hospital, Dallas, TX, USA.
9Arkansas Children’s Hospital, Little Rock, AK, USA.
10Children’s Healthcare of Atlanta, Atlanta, GA, USA.
11Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA.
12Children’s Hospital Wisconsin, Milwaukee, WI, USA.
13Phoenix Children’s Hospital, Phoenix, AZ, USA.
14Children’s of Alabama, Birmingham, AB, USA.
15Riley Hospital for Children, Indianapolis, IN, USA.
16LeBonheur Children’s Hospital, Memphis, TN, USA.
17Medical University of South Carolina, Charleston, SC, USA.
18University of Michigan, Ann Arbor, MI, USA.
Funding
This study was supported by the Heart Institute Research Core (HIRC) at Cincinnati Children’s Hospital with additional funding from Castin’ ‘N Catchin’ Charity Organization. The funding sources for this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
DH and KMG conceptualized and designed the study, assisted with data analysis and interpretation, drafted the initial manuscript, and reviewed and revised the manuscript. HZ performed statistical analysis and reviewed and revised the manuscript. JA conceptualized and designed the study, provided support and mentorship, and reviewed and revised the manuscript. All other authors assisted with the design of the study, data interpretation, and reviewed and revised the manuscript for important intellectual content. All authors approve the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
All authors report no real or perceived conflicts of interest that could affect the study design, collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication. For full disclosure, we provide the additional list of authors’ other funding not directly related to this study. DA is a consultant for Baxter, Nuwellis, Medtronic Bioporto, and Seastar. His institution receives grant funding for education and research that is not related to this project from NIH, Baxter, Nuwellis, Medtronic, Bioporto, and Seastar. He has patents pending on inventions to improve the kidney care of neonates. He is the Founder and Chief Scientific Officer for Zorro-Flow. KMG receives consultant fees from Bioporto Diagnostics and Potrero Medical. SLG is a consultant for Baxter, Medtronic, Bioporto, and Protrero. He is the founder and Chief Scientific Officer for Medibeacon. No other disclosures were reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasson, D.C., Alten, J.A., Bertrandt, R.A. et al. Persistent acute kidney injury and fluid accumulation with outcomes after the Norwood procedure: report from NEPHRON. Pediatr Nephrol 39, 1627–1637 (2024). https://doi.org/10.1007/s00467-023-06235-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06235-y