Abstract
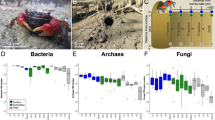
Habitat edge effects can have profound impacts on biodiversity throughout terrestrial and aquatic biomes. Yet, few studies have examined how habitat edge effects impact the spatial patterning of sediment properties and microbial communities, especially in coastal ecosystems. Coastal salt marshes throughout the world are being transformed by sea level rise; high-marsh, flood-intolerant species, such as Spartina patens, are being fragmented and replaced by low-marsh, flood-tolerant species, such as Spartina alterniflora. The consequences of these habitat transformations on fungal communities remain unclear. Thus, we sought to identify how habitat edge effects, alongside changing plant community dynamics, impact the spatial patterning of fungal communities associated with ubiquitous Spartina species. We analyzed 26 Spartina patens patches: 13 pure monocultures and 13 mixed patches with Spartina alterniflora infiltration. We measured patch characteristics, plant characteristics, sediment physicochemical properties, and sediment fungal communities. We found that habitat edge effects structured sediment and plant properties in both pure and mixed patches. However, habitat edge effects only structured fungal community composition in mixed patches, counter to expectations. These results indicate that changing plant community dynamics driven by sea level rise can exacerbate habitat edge effects in coastal ecosystems. Least discriminant analysis and co-occurrence networks further revealed unique taxa and network structures between pure and mixed patches and between interiors and edges. In sum, we found that habitat transformation of coastal salt marshes driven by global change impacts the spatial dynamics of sediment and fungal properties.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bass J, Granse D, Hache I, Jensen K, Karius V, Minden V, Stock M, Suchrow S, Kleyer M (2022) Plant traits affect vertical accretion of salt marshes. Estuar Coast Shelf Sci 276:108010
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67:1–48
Berry D, Widder S (2014) Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Frontiers in Microbiology 5:1–14
Bertness MD (1991) Zonation of Spartina patens and Spartina alterniflora in New England salt marsh. Ecology 72:138–148
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. National Biotechnology 37:852–857
Boström C, Pittman SJ, Simenstad C, Kneib RT (2011) Seascape ecology of coastal biogenic habitats: advances, gaps, and challenges. Marine Ecology Progress Series 427:191–217
Buchan A, Newell SY, Butler M, Biers EJ, Hollibaugh JT, Moran MA (2003) Dynamics of Bacterial and Fungal communities on Decaying Salt Marsh Grass. Applied and Environmental Microbiology 69:6676–6687
Buchan A, Newell SY, Moreta JL, Moran MA (2002) Analysis of internal transcribed spacer (ITS) regions of rRNA genes in fungal communities in a southeastern US salt marsh. Microbial Ecology 43:329–340
Burke DJ, Hamerlynck EP, Hahn D (2002) Effect of arbuscular mycorrhizae on soil microbial populations and associated plant performance of the salt marsh grass Spartina patens. Plant Soil 239:141–154
Burke DJ, Hamerlynck EP, Hahn D (2003) Interactions between the salt marsh grass Spartina patens, arbuscular mycorrhizal fungi and sediment bacteria during the growing season. Soil Biology and Biochemistry 35:501–511
Cahoon DR, McKee KL, Morris JT (2021) How plants influence resilience of Salt Marsh and Mangrove wetlands to Sea-Level rise. Estuaries Coasts 44:883–898
Calabon MS, Jones EBG, Promputtha I, Hyde KD (2021) Fungal biodiversity in Salt Marsh ecosystems. Journal of Fungi 7:648
Calado M, Carvalho L, Barata L, Pang M (2019) Potential roles of marine fungi in the decomposition process of standing stems and leaves of Spartina maritima. Mycologia 111:371–383
Calado M, Carvalho L, Pang L, Barata K-L (2015) Diversity and ecological characterization of sporulating higher Filamentous Marine Fungi Associated with Spartina maritima (Curtis) Fernald in two Portuguese salt marshes. Microbial Ecology 70:612–633
Campbell AD, Wang Y (2020) Salt marsh monitoring along the Mid-atlantic coast by Google Earth Engine enabled time series. PLoS ONE 15:e0229605
Cao Y, Dong Q, Wang D, Zhang P, Liu Y, Niu C (2022) microbiomeMarker: an R/Bioconductor package for microbiome marker identification and visualization. Bioinformatics 38:4027–4029
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7:335–336
Carroll JM, Keller DA, Furman BT, Stubler AD (2019) Rough around the edges: lessons learned and future directions in Marine Edge effects studies. Current Landscape Ecology Reports 4:91–102
Chen Y, Li Y, Thompson C, Wang X, Cai T, Chang Y (2018) Differential sediment trapping abilities of mangrove and saltmarsh vegetation in a subtropical estuary. Geomorphology 318:270–282
Chung NC, Miasojedow B, Startek M, Gambin A (2019) Jaccard/Tanimoto similarity test and estimation methods for biological presence-absence data. BMC Bioinformatics 20:644
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18:117–143
d’Entremont TW, López-Gutiérrez JC, Walker AK (2018) Examining arbuscular mycorrhizal fungi in saltmarsh hay (Spartina patens) and smooth cordgrass (Spartina alterniflora) in the Minas Basin, Nova Scotia. Northeastern Naturalist 25:72–86
d’Entremont TW, Migicovsky Z, López-Gutiérrez JC, Walker AK (2021) Saltmarsh rhizosphere fungal communities vary by sediment type and dominant plant species cover in Nova Scotia, Canada. Environmental Microbiology Reports 13:458–463
Denno RF (1977) Comparison of the assemblages of sap-feeding insects (Homoptera-Hemiptera) inhabiting two structurally different salt marsh grasses in the genus Spartina. Environmental Entomology 6:359–372
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics 34:487–515
Faith DP, Minchin PR, Belbin L (1987) Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 69:57–68
Fan K, Weisenhorn P, Gilbert JA, Chu H (2018) Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biology and Biochemistry 125:251–260
Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, Huttenhower C (2012) Microbial Co-occurrence relationships in the human microbiome. PLoS Computational Biology 8:e1002606
Fletcher RJ Jr, Didham RK, Banks-Leite C, Barlow J, Ewers RM, Rosindell J, Holt RD, Gonzalez A, Pardini R, Damschen EI (2018) Is habitat fragmentation good for biodiversity? Biological Conservation 226:9–15
Grilli G, Longo S, Huais P, Pereyra M, Verga E, Urcelay C, Galetto L (2017) Fungal diversity at fragmented landscapes: synthesis and future perspectives. Current Opinion in Microbiology 37:161–165
Grilli G, Urcelay C, Galetto L (2012) Forest fragment size and nutrient availability: complex responses of mycorrhizal fungi in native–exotic hosts. Plant Ecology 213:155–165
Grilli G, Urcelay C, Galetto L, Davison J, Vasar M, Saks Ü, Jairus T, Öpik M (2015) The composition of arbuscular mycorrhizal fungal communities in the roots of a ruderal forb is not related to the forest fragmentation process. Environmental Microbiology 17:2709–2720
Jaccard P (1912) The distribution of the flora in the alpine zone. New Phytologist 11:37–50
Jiang X-T, Peng X, Deng G-H, Sheng H-F, Wang Y, Zhou H-W, Tam NF-Y (2013) Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a Mangrove Wetland. Microbial Ecology 66:96–104
Kearns PJ, Bulseco-McKim AN, Hoyt H, Angell JH, Bowen JL (2019) Nutrient Enrichment alters Salt Marsh Fungal communities and promotes putative fungal denitrifiers. Microbial Ecology 77:358–369
Kiesewetter KN, Afkhami ME (2021) Microbiome-mediated effects of habitat fragmentation on native plant performance. New Phytologist 232:1823–1838
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lücking R, Martín MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüßler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson K-H (2013) Towards a unified paradigm for sequence-based identification of fungi. Molecular Ecology 22:5271–5277
Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R (2012) Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Current Protocols in Microbiology 27:1E – 5
Laurance WF, Nascimento HEM, Laurance SG et al (2007) Habitat Fragmentation, Variable Edge effects, and the Landscape-Divergence hypothesis. PLoS ONE 2:e1017. https://doi.org/10.1371/journal.pone.0001017
Laurance WF, Yensen E (1991) Predicting the impacts of edge effects in fragmented habitats. Biological Conservationa 55:77–92
Levine JM, Brewer JS, Bertness MD (1998) Nutrients, competition and plant zonation in a New England salt marsh. Journal of Ecology 86:285–292
Lippok D, Beck SG, Renison D, Hensen I, Apaza AE, Schleuning M (2014) Topography and edge effects are more important than elevation as drivers of vegetation patterns in a neotropical montane forest. Journal of Vegetation Science 25:724–733
Lynum CA, Bulseco AN, Dunphy CM, Osborne SM, Vineis JH, Bowen JL (2020) Microbial Community response to a Passive Salt Marsh Restoration. Estuaries and Coasts 43:1439–1455
Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM (2014) Taxonomical and functional microbial community selection in soybean rhizosphere. ISME Journal 8:1577–1587
Mohamed DJ, Martiny JB (2011) Patterns of fungal diversity and composition along a salinity gradient. ISME Journal 5:379–388
Newell SY, Porter D, Lingle WL (1996) Lignocellulolysis by ascomycetes (fungi) of a saltmarsh grass (smooth cordgrass). Microscopy Research and Technique 33:32–46
NJDEP (New Jersey Department of Environmental Protection) (2022) NJDEP Bureau of GIS. NJDEP open data. Available at: New Jersey Department of Environmental Protection. https://gisdata-njdep.opendata.arcgis.com/.
Nilsson RH, Larsson K-H, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Glöckner FO, Tedersoo L, Saar I, Kõljalg U, Abarenkov K (2019) The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Research 47:D259–D264
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Package ‘vegan’. Community ecology package, version 2:1–295
Onufrak A, Rúa MA, Hossler K (2020) The missing metric: an evaluation of fungal importance in wetland assessments. Wetlands 40:825–838
Pennings SC, Selig ER, Houser LT, Bertness MD (2003) Geographic Variation in positive and negative interactions among Salt Marsh plants. Ecology 84:1527–1538
Perreault R, Laforest-Lapointe I (2022) Plant-microbe interactions in the phyllosphere: facing challenges of the anthropocene. The ISME Journal 16:339–345
Püttker T, Crouzeilles R, Almeida-Gomes M, Schmoeller M, Maurenza D, Alves-Pinto H, Pardini R, Vieira MV, Banks-Leite C, Fonseca CR (2020) Indirect effects of habitat loss via habitat fragmentation: a cross-taxa analysis of forest-dependent species. Biological Conservation 241:108368
Raghukumar S (2017) The Salt Marsh Ecosystem. Pages 87–101 Fungi in Coastal and Oceanic Marine Ecosystems. Springer, Cham
Ramírez-Viga TK, Aguilar R, Castillo-Argüero S, Chiappa-Carrara X, Guadarrama P, Ramos-Zapata J (2018) Wetland plant species improve performance when inoculated with arbuscular mycorrhizal fungi: a meta-analysis of experimental pot studies. Mycorrhiza 28:477–493
Ries L, Murphy SM, Wimp GM, Fletcher RJ (2017) Closing persistent gaps in knowledge about edge ecology. Current Landscape Ecology Reports 2:30–41
Rillig MC, Ryo M, Lehmann A, Aguilar-Trigueros CA, Buchert S, Wulf A, Iwasaki A, Roy J, Yang G (2019) The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 366:886–890
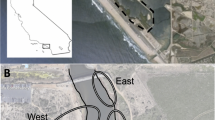
Rippel TM, Minsavage-Davis CD, Shirey V, Wimp GM (2023) Simple machine learning with aerial imagery reveals severe loss of a salt Marsh Foundation species. Estuaries and Coasts. https://doi.org/10.1007/s12237-023-01192-z. (Last accessed 24/04/2023)
Rippel TM, Mooring EQ, Tomasula J, Wimp GM (2020) Habitat edge effects decrease litter accumulation and increase litter decomposition in coastal salt marshes. Landscape Ecology 35:2179–2190
Ruwanza S (2018) The edge effect on plant diversity and soil properties in abandoned fields targeted for ecological restoration. Sustainability 11:140
Smith S (2019) Phylosmith: An R-package for reproducible and efficient microbiome analysis with phyloseq-objects. Journal of Open Source Software 4(48):1442. https://doi.org/10.21105/joss.01442
Snedden GA, Cretini K, Patton B (2015) Inundation and salinity impacts to above- and belowground productivity in Spartina patens and Spartina alterniflora in the Mississippi River deltaic plain: implications for using river diversions as restoration tools. Ecological Engineering 81:133–139
Su N, Jarvie S, Yan Y, Gong X, Li F, Han P, Zhang Q (2022) Landscape context determines soil fungal diversity in a fragmented habitat. CATENA 213:106163
Su G, Morris JH, Demchak B, Bader GD (2014) Biological Network Exploration with Cytoscape 3. Current Protocols in Bioinformatics 47:8.13.1–8.13.24
Team RC (2021) R: A language and environment for statistical computing (Version 4.0. 5)[Computer software]. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Vinent OD, Herbert ER, Coleman DJ, Himmelstein JD, Kirwan ML (2021) Onset of runaway fragmentation of salt marshes. One Earth 4:506–516
Visser J, Sandy E (2009) The effects of flooding on four common Louisiana Marsh plants. Gulf of Mexico Science 27(1). https://aquila.usm.edu/goms/vol27/iss1/3
de Vries FT, Griffiths RI, Bailey M, Craig H, Girlanda M, Gweon HS, Hallin S, Kaisermann A, Keith AM, Kretzschmar M, Lemanceau P, Lumini E, Mason KE, Oliver A, Ostle N, Prosser JI, Thion C, Thomson B, Bardgett RD (2018) Soil bacterial networks are less stable under drought than fungal networks. Nature Communications 9:3033
Wang H, Wu C, Zhang H, Xiao M, Ge T, Zhou Z, Liu Y, Peng S, Peng P, Chen J (2022) Characterization of the belowground microbial community and co-occurrence networks of Tobacco plants infected with bacterial wilt Disease. World Journal of Microbiology and Biotechnology 38:155
Watson EB, Szura K, Wigand C, Raposa KB, Blount K, Cencer M (2016) Sea level rise, drought and the decline of Spartina patens in New England marshes. Biological Conservation 196:173–181
Welsh AK, Burke DJ, Hamerlynck EP, Hahn D (2010) Seasonal analyses of arbuscular mycorrhizae, nitrogen-fixing bacteria and growth performance of the salt marsh grass Spartina patens. Plant Soil 330:251–266
Wimp GM, Murphy SM (2021) Habitat edges alter arthropod community composition. Landscape Ecology 36:2849–2861
Wu L, Feng S, Nie Y, Zhou J, Yang Z, Zhang J (2015) Soil cellulase activity and fungal community responses to wetland degradation in the Zoige Plateau, China. Journal of Mountain Science 12:471–482
Yang W, Zhang D, Cai X, Xia L, Luo Y, Cheng X, An S (2019) Significant alterations in soil fungal communities along a chronosequence of Spartina alterniflora invasion in a Chinese Yellow Sea coastal wetland. Science of the Total Environment 693:133548
Yeager LA, Estrada J, Holt K, Keyser SR, Oke TA (2020) Are habitat fragmentation effects stronger in marine systems? A review and meta-analysis. Current Landscape Ecology Reports 5:58–67
Zhang G, Bai J, Tebbe CC, Huang L, Jia J, Wang W, Wang X, Yu L, Zhao Q (2021) Spartina alterniflora invasions reduce soil fungal diversity and simplify co-occurrence networks in a salt marsh ecosystem. Science of the Total Environment 758:143667
Zhang B, Zhang J, Liu Y, Shi P, Wei G (2018) Co-occurrence patterns of soybean rhizosphere microbiome at a continental scale. Soil Biology and Biochemistry 118:178–186
Acknowledgements
We thank Billie Maguire, Jewel Tomasula, Kayla Nocon, Sophia Kim, and Collette Hong for lab and field work help.
Funding
This work was supported by Cosmos’ Club Foundation, Wetland Foundation, and Georgetown University with grants to Tyler M. Rippel.
Author information
Authors and Affiliations
Contributions
Study conception and design was conceived by Tyler M. Rippel and Gina M. Wimp. Material preparation and data collection were performed by Cathilyn L. Mcintosh, Shannon. M. Murphy, and Tyler M. Rippel. Data analysis was performed by Tyler M. Rippel. Data interpretation was performed by Alexandra L. DeCandia, Melissa A. Collier, and Tyler M. Rippel. The first draft of the manuscript was written by Tyler M. Rippel, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rippel, T.M., DeCandia, A.L., Collier, M.A. et al. Habitat Characteristics and Plant Community Dynamics Impact the Diversity, Composition, and Co-occurrence of Sediment Fungal Communities. Wetlands 44, 3 (2024). https://doi.org/10.1007/s13157-023-01756-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-023-01756-6