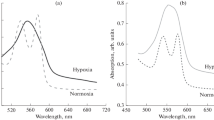
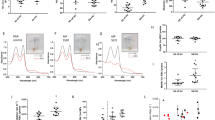
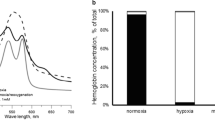
Abstract—Metabolic stress caused by a lack of glucose significantly affects the state of red blood cells, where glycolysis is the main pathway for the production of ATP. Hypoglycemia can be both physiological (occurring during fasting and heavy physical exertion) and pathological (accompanying a number of diseases, such as diabetes mellitus). In this study, we have characterized the state of isolated erythrocytes under metabolic stress caused by the absence of glucose. It was established that 24 h of incubation of the erythrocytes in a glucose-free medium to simulate blood plasma led to a two-fold decrease in the ATP level into them. The cell size, as well as intracellular sodium concentration increased. These findings could be the result of a disruption in ion transporter functioning because of a decrease in the ATP level. The calcium level remained unchanged. With a lack of glucose in the medium of isolated erythrocytes, there was no increase in ROS and a significant change in the level of nitric oxide, while the level of the main low-molecular weight thiol of cells, glutathione (GSH) decreased by almost 2 times. It was found that the metabolic stress of isolated red blood cells induced hemoglobin glutathionylation despite the absence of ROS growth. The cause was the lack of ATP, which led to a decrease in the level of GSH because of the inhibition of its synthesis and, probably, due to a decrease in the NADPH level required for glutathione (GSSG) reduction and protein deglutathionylation. Thus, erythrocyte metabolic stress induced hemoglobin glutathionylation, which is not associated with an increase in ROS. This may have an important physiological significance, since glutathionylation of hemoglobin changes its affinity for oxygen.






Similar content being viewed by others
REFERENCES
Franco R.S. 2012. Measurement of red cell lifespan and aging. Transfus. Med. Hemother. 39, 302–307. https://doi.org/10.1159/000342232
Moura P.L., Hawley B.R., Mankelow T.J., Griffiths R.E., Dobbe J.G.G., Streekstra G.J., Anstee D.J., Satchwell T.J., Toye A.M. 2018. Non-muscle myosin II drives vesicle loss during human reticulocyte maturation. Haematologica. 103, 1997–2007. https://doi.org/10.3324/haematol.2018.199083
Ovchynnikova E., Aglialoro F., Von Lindern M., Van Den Akker E. 2018. The shape shifting story of reticulocyte maturation. Front. Physiol. 9, 829. https://doi.org/10.3389/fphys.2018.00829
Mohanty J.G., Nagababu E., Rifkind J.M. 2014. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 5, 84. https://doi.org/10.3389/fphys.2014.00084
Tang H., Ho H., Wu P., Chen S., Kuypers F.A., Cheng M., Chiu D.T. 2015. Inability to maintain GSH pool in G6PD-deficient red cells causes futile AMPK activation and irreversible metabolic disturbance. Antioxid. Redox Signal. 22, 744–759. https://doi.org/10.1089/ars.2014.6142
Mamillapalli C., Tentu R., Jain N.K., Bhandari R. 2019. COPD and type 2 diabetes. Curr. Respir. Med. Rev. 15, 112–119. https://doi.org/10.2174/1573398X15666190211155640
Vasileiadis I., Alevrakis E., Ampelioti S., Vagionas D., Rovina N., Koutsoukou A. 2019. Acid-base disturbances in patients with asthma: A literature review and comments on their pathophysiology. J. Clin. Med. 8, 563, https://doi.org/10.3390/jcm8040563
Sircar M., Bhatia A., Munshi M. 2016. Review of hypoglycemia in the older adult: Clinical implications and management. Can. J. Diabetes. 40, 66–72. https://doi.org/10.1016/j.jcjd.2015.10.004
UK Hypoglycaemia study group 2007. Risk of hypoglycaemia in types 1 and 2 diabetes: Effects of treatment modalities and their duration. Diabetologia. 50, 1140–1147. https://doi.org/10.1007/s00125-007-0599-y
van Wijk R., van Solinge W.W. 2005. The energy-less red blood cell is lost: Erythrocyte enzyme abnormalities of glycolysis. Blood. 106, 4034–4042. https://doi.org/10.1182/blood-2005-04-1622
Pandey K.B., Rizvi S.I. 2011. Biomarkers of oxidative stress in red blood cells. Biomed. Pap. 155, 131–136. https://doi.org/10.5507/bp.2011.027
Miwa S. 1983. Hereditary disorders of red cell enzymes in the Embden–Meyerhof pathway. Am. J. Hematol. 14, 381–391. https://doi.org/10.1002/ajh.2830140410
Koralkova P., van Solinge W.W., van Wijk R. 2014. Rare hereditary red blood cell enzymopathies associated with hemolytic anemia—pathophysiology, clinical aspects, and laboratory diagnosis. Int. J. Lab. Hematol. 36, 388–397. https://doi.org/10.1111/ijlh.12223
Lang F., Abed M., Lang E., Föller M. 2014. Oxidative stress and suicidal erythrocyte death. Antioxid. Redox Signal. 21, 138–153. https://doi.org/10.1089/ars.2013.5747
Gilbert H.F. 2006. Molecular and cellular aspects of thiol-disulfide exchange. Adv. Enzymol. Relat. Areas Mol. Biol. 63, 69–172.
Poluektov Y.M., Petrushanko I.Yu., Undrovinas N.A., Lakunina V.A., Khapchaev A.Y., Kapelko V.I., Abra-mov A.A., Lakomkin V.L., Novikov M.S., Shirinsky V.P. 2019. Glutathione-related substances maintain cardiomyocyte contractile function in hypoxic conditions. Sci. Rep. 9, 4872. https://doi.org/10.1038/s41598-019-41266-2
Mieyal J.J., Gallogly M.M., Qanungo S., Sabens E.A., Shelton M.D. 2008. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid. Redox Signal. 10, 1941–1988. https://doi.org/10.1089/ars.2008.2089
Petrushanko I.Yu., Yakushev S., Mitkevich V.A., Kamanina Y.V., Ziganshin R.H., Meng X., Anash-kina A.A., Makhro A., Lopina O.D., Gassmann M. 2012. S-glutathionylation of the Na,K-ATPase catalytic α subunit is a determinant of the enzyme redox sensitivity. J. Biol. Chem. 287, 32195–32205. https://doi.org/10.1074/jbc.M112.391094
Giustarini D., Dalle-Donne I., Milzani A., Braconi D., Santucci A., Rossi R. 2019. Membrane skeletal protein S-glutathionylation in human red blood cells as index of oxidative stress. Chem. Res. Toxicol. 32, 1096–1102. https://doi.org/10.1021/acs.chemrestox.8b00408
Brinkmann C., Neumann E., Blossfeld J., Frese S., Orthmann P., Montiel G., Bloch W., Brixius K. 2011. Influence of glycemic status and physical fitness on oxidative stress and the peroxiredoxin system in the erythrocytes of non-insulin-dependent type 2 diabetic men. Exp. Clin. Endocrinol. Diabetes. 119, 559–564. https://doi.org/10.1055/s-0031-1279712
Yousefzade G., Nakhaee A. 2012. Insulin-induced hypoglycemia and stress oxidative state in healthy people. Acta Diabetol. 49, 81–85. https://doi.org/10.1007/s00592-011-0311-z
Pompeo G., Girasole M., Cricenti A., Boumis G., Bellelli A., Amiconi S. 2010. Erythrocyte death in vitro induced by starvation in the absence of Ca2+. Biochim. Biophys. Acta. 1798, 1047–1055. https://doi.org/10.1016/j.bbamem.2010.02.002
Nemkov T., Qadri S.M., Sheffield W.P., D’Alessandro A. 2020. Decoding the metabolic landscape of pathophysiological stress-induced cell death in anucleate red blood cells. Blood Transfus. 130, 130‒142. https://doi.org/10.2450/2020.0256-19
Van Cromvoirt A.M., Fenk S., Sadafi A., Melni-kova E.V., Lagutkin D.A., Dey K., Petrushanko I.Yu., Hegemann I., Goede J.S., Bogdanova A. 2021. Donor age and red cell age contribute to the variance in lorrca indices in healthy donors for next generation ektacytometry: A pilot study. Front. Physiol. 12, 639722. https://doi.org/10.3389/fphys.2021.639722
Petrushanko I., Bogdanov N., Bulygina E., Grenacher B., Leinsoo T., Boldyrev A., Gassmann M., Bogdanova A. 2006. Na-K-ATPase in rat cerebellar granule cells is redox sensitive. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, 916–925. https://doi.org/10.1152/ajpregu.00038.2005
Petrushanko I.Yu., Bogdanov N.B., Lapina N., Boldyrev A.A., Gassmann M., Bogdanova A.Yu. 2007. Oxygen-induced regulation of Na/K ATPase in cerebellar granule cells. J. Gen. Physiol. 130, 389–398. https://doi.org/10.1085/jgp.200709783
Mitkevich V.A., Kretova O.V., Petrushanko I.Yu., Burnysheva K.M., Sosin D.V., Simonenko O.V., Ilinskaya O.N., Tchurikov N.A., Makarov A.A. 2013. Ribonuclease binase apoptotic signature in leukemic kasumi-1 cells. Biochimie. 95, 1344–1349. https://doi.org/10.1016/j.biochi.2013.02.016
Yamamoto A., Saito N., Yamauchi Y., Takeda M., Ueki S., Itoga M., Kojima K., Kayaba H. 2014. Flow cytometric analysis of red blood cell osmotic fragility. SLAS Technol. 19, 483–487. https://doi.org/10.1177/2211068214532254
Slatinskaya O.V., Zaripov P.I., Brazhe N.A., Petrushanko I.Yu., Maksimov G.V. 2022. Changes in the conformation and distribution of hemoglobin in the erythrocyte upon inhibition of Na+/K+-ATPase activity. Biophysics. 67, 726–733. https://doi.org/10.1134/S0006350922050189
Makhro A., Huisjes R., Verhagen L.P., Mañú-Pereira M. del M., Llaudet-Planas E., Petkova-Kirova P., Wang J., Eichler H., Bogdanova A., van Wijk R. 2016. Red cell properties after different modes of blood transportation. Front. Physiol. 7, 288. https://doi.org/10.3389/fphys.2016.00288
Fedorov D.A., Sidorenko S.V., Yusipovich A.I., Parshina E.Y., Tverskoi A.M., Abramicheva P.A., Maksi-mov G.V., Orlov S.N., Lopina O.D., Klimanova E.A. 2021. Na\(_{i}^{ + }\)/K\(_{i}^{ + }\) imbalance contributes to gene expression in endothelial cells exposed to elevated NaCl. Heliyon. 7, e08088. https://doi.org/10.1016/j.heliyon.2021.e0808810.1016/j.heliyon.2021.e08088
Zhang R., Xiang Y., Ran Q., Deng X., Xiao Y., Xiang L., Li Z. 2014. Involvement of calcium, reactive oxygen species, and ATP in hexavalent chromium-induced damage in red blood cells. Cell. Physiol. Biochem. 34, 1780–1791. https://doi.org/10.1159/000366378
Suh S., Kim J.H. 2015. Glycemic variability: How do we measure it and why is it important? Diabetes Metab. J. 39, 273‒282. https://doi.org/10.4093/dmj.2015.39.4.273
Cryer P.E., Axelrod L., Grossman A.B., Heller S.R., Montori V.M., Seaquist E.R., Service F.J. 2009. Evaluation and management of adult hypoglycemic disorders: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 94, 709–728. https://doi.org/10.1210/jc.2008-1410
Szadkowska A., Czyżewska K., Pietrzak I., Mianowska B., Jarosz-Chobot P., Myśliwiec M. 2018. Hypoglycaemia unawareness in patients with type 1 diabetes. Pediatr. Endocrinol. Diabetes Metab. 24, 126–134. https://doi.org/10.5114/pedm.2018.80994
Silbert R., Salcido-Montenegro A., Rodriguez-Gutierrez R., Katabi A., McCoy R.G. 2018. Hypoglycemia among patients with type 2 diabetes: Epidemiology, risk factors, and prevention strategies. Curr. Diab. Rep. 18, 53. https://doi.org/10.1007/s11892-018-1018-0
Vega-Cano S., Cordero-Vázquez E., Mestre-Torres J. 2021. Hipoglucemia como forma de presentación de infiltración hipofisaria por un linfoma. Med. Clínica. 156, 362–363. https://doi.org/10.1016/j.medcli.2020.01.040
Wang J., Zhu C.-K., Yu J.-Q., Tan R., Yang P.-L. 2021. Hypoglycemia and mortality in sepsis patients: A systematic review and meta-analysis. Heart Lung. 50, 933–940. https://doi.org/10.1016/j.hrtlng.2021.07.017
Salehi M., Vella A., McLaughlin T., Patti M.-E. 2018. Hypoglycemia after gastric bypass surgery: Current concepts and controversies. J. Clin. Endocrinol. Metab. 103, 2815–2826. https://doi.org/10.1210/jc.2018-00528
Amiel S.A. 2021. The consequences of hypoglycaemia. Diabetologia. 64, 963–970. https://doi.org/10.1007/s00125-020-05366-3
Papachristoforou E., Lambadiari V., Maratou E., Makrilakis K. 2020. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J. Diabetes Res. 2020, 7489795. https://doi.org/10.1155/2020/7489795
Rogers T.B., Lokuta A.J. 1994. Angiotensin II signal transduction pathways in the cardiovascular system. Trends Cardiovasc. Med. 4, 110–116. https://doi.org/10.1016/1050-1738(94)90062-0
Kosower N.S., Zipser Y., Faltin Z. 1982. Membrane thiol-disulfide status in glucose-6-phosphate dehydrogenase deficient red cells. relationship to cellular glutathione. Biochim. Biophys. Acta. 691, 345–352. https://doi.org/10.1016/0005-2736(82)90424-2
Craescu C.T., Poyart C., Schaefferll C., Garel M.-C., Kisterp J., Beuzard Y. 1986. Covalent binding of glutathione to hemoglobin. II. Functional consequences and structural changes reflected in NMR spectra. J. B-iol. Chem. 261, 14710–14716.
Metere A., Iorio E., Scorza G., Camerini S., Casella M., Crescenzi M., Minetti M., Pietraforte D. 2014. Carbon monoxide signaling in human red blood cells: Evidence for pentose phosphate pathway activation and protein deglutathionylation. Antioxid. Redox Signal. 20, 403–416. https://doi.org/10.1089/ars.2012.5102
Colombo G., Dalle-Donne I., Giustarini D., Gagliano N., Portinaro N., Colombo R., Rossi R., Milzani A. 2010. Cellular redox potential and hemoglobin S-glutathionylation in human and rat erythrocytes: A comparative study. Blood Cells. Mol. Dis. 44, 133–139. https://doi.org/10.1016/j.bcmd.2009.11.005
Perutz M.F. 1970. Stereochemistry of cooperative effects in haemoglobin: Haem–haem interaction and the problem of allostery. Nature. 228, 726–734. https://doi.org/https://doi.org/10.1038/228726a0
Rubino F.M. 2021. The redox potential of the β-93-cysteine thiol group in human hemoglobin estimated from in vitro oxidant challenge experiments. Molecules. 26, 2528. https://doi.org/10.3390/molecules26092528
Fenk S., Melnikova E.V., Anashkina A.A., Po-luektov Y.M., Zaripov P.I., Mitkevich V.A., Tkachev Y.V., Kaestner L., Minetti G., Mairbäurl H. 2022. Hemoglobin is an oxygen-dependent glutathione buffer adapting the intracellular reduced glutathione levels to oxygen availability. Redox Biol. 58, 102535. https://doi.org/10.1016/j.redox.2022.102535
Chen H.-J.C., Lin W.-P., Chiu S.-D., Fan C.-H. 2014. Multistage mass spectrometric analysis of human hemoglobin glutathionylation: Correlation with cigarette smoking. Chem. Res. Toxicol. 27, 864–872. https://doi.org/10.1021/tx5000359
Collins J.-A., Rudenski A., Gibson J., Howard L., O’Driscoll R. 2015. Relating oxygen partial pressure, saturation and content: The haemoglobin–oxygen dissociation curve. Breathe (Sheff). 11, 194–201. https://doi.org/10.1183/20734735.001415
Ghezzi P. 2013. Protein glutathionylation in health and disease. Biochim. Biophys. Acta. 1830, 3165–3172. https://doi.org/10.1016/j.bbagen.2013.02.009
Radosinska J., Vrbjar N. 2021. Erythrocyte deformability and Na,K-ATPase activity in various pathophysiological situations and their protection by selected nutritional antioxidants in humans. Int. J. Mol. Sci. 22, 11924. https://doi.org/10.3390/ijms222111924
Funding
This research was funded by the Russian Science Foundation grant no. 19-14-00374.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All procedures performed in this work comply with the ethical standards of the institutional research ethics committee and the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. Informed consent was obtained from all patients for medical examination, including blood sampling.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaripov, P.I., Kuleshova, Y.D., Poluektov, Y.M. et al. Metabolic Stress of Red Blood Cells Induces Hemoglobin Glutathionylation. Mol Biol 57, 1176–1185 (2023). https://doi.org/10.1134/S0026893323060225
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893323060225