Abstract
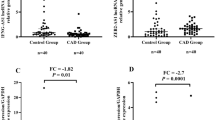
Peripheral blood biomarkers are of particular importance to diagnose certain diseases including coronary artery disease (CAD) due to their non-invasiveness. Investigating the expression of noncoding RNAs (ncRNAs) paves the way to early disease diagnosis, prognosis, and treatment. Consequently, in this research, we aimed to investigate a panel of ncRNAs as potential biomarkers in patients with coronary artery disease. Two different groups have been designed (control and CAD). All participants were subjected to interviews and clinical examinations. Peripheral blood samples were collected, and plasma was extracted. At the same time, target ncRNAs have been selected based on literature review and bioinformatic analysis, and later they underwent investigation using quantitative real-time PCR. The selected panel encompassed the long non-coding RNAs (lncRNAs) MEG3, TUG1, and SRA1, and one related microRNA (miRNA): hsa-miR-21-3p. We observed statistically significant upregulation in MEG3, TUG1, and hsa-miR21-3p in CAD patients compared to control participants (p-value < 0.01). Nevertheless, SRA1 exhibited downregulation with no statistical significance (p-value > 0.05). All ncRNAs under study displayed a significantly strong correlation with disease incidence, age, and smoking. Network construction revealed a strong relationship between MEG3 and TUG1. ROC analysis indicated high potentiality for hsa-miR-21-3p to be a promising biomarker for CAD. Moreover, MEG3 and TUG1 displayed distinguished diagnostic discrimination but less than hsa-miR-21-3p, all of them exhibited strong statistical significance differences between CAD and control groups. Conclusively, this research pinpointed that MEG3, TUG1, and hsa-miR-21-3p are potential biomarkers of CAD incidence and diagnosis.




Similar content being viewed by others
REFERENCES
Malakar A.K., Choudhury D., Halder B., Paul P., Uddin A., Chakraborty S. 2019. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell Physiol. 234 (10), 16812–16823. https://doi.org/10.1002/jcp.28350
Wan Q., Qian S., Huang Y., Zhang Y., Peng Z., Li Q., Shu B., Zhu L., Wang M. 2020. Drug discovery for coronary artery disease. Adv. Exp. Med. Biol. 1177, 297–339.
Hunt S.A., Abraham W.T., Chin M.H., Feldman A.M., Francis G.S., Ganiats T.G., Jessup M., Konstam M.A., Mancini D.M., Michl K., Oates J.A., Rahko P.S., Silver M.A., Stevenson L.W., Yancy C.W. 2009. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the American college of cardiology foundation/American heart association task force on practice guidelines developed in collaboration with the International society for heart and lung transplantation. J. Am. Coll. Cardiol. 53 (15), e1–e90. https://doi.org/10.1016/j.jacc.2008.11.013
Netto J., Teren A., Burkhardt R., Willenberg A., Beutner F., Henger S., Schuler G., Thiele H., Isermann B., Thiery J., Scholz M., Kaiser T. 2022. Biomarkers for non-invasive stratification of coronary artery disease and prognostic impact on long-term survival in patients with stable coronary heart disease. Nutrients. 14 (16), 3433. https://doi.org/10.3390/nu14163433
Parsanathan R., Jain S.K. 2020. Novel invasive and noninvasive cardiac-specific biomarkers in obesity and cardiovascular diseases. Metab. Syndr. Relat. Disord. 18 (1), 10–30. https://doi.org/10.1089/met.2019.0073
Cardona-Monzonís A., García-Giménez J.L., Mena-Mollá S., Pareja-Galeano H., de la Guía-Galipienso F., Lippi G., Pallardó F.V., Sanchis-Gomar F. 2020. Non-coding RNAs and coronary artery disease. In Advances in Experimental Medicine and Biology. Vol. 1229. Xiao J., Ed. Singapore: Springer, 273–285. https://doi.org/10.1007/978-981-15-1671-9_16
Poller W., Dimmeler S., Heymans S., Zeller T., Haas J., Karakas M., Leistner D.M., Jakob P., Nakagawa S., Blankenberg S., Engelhardt S., Thum T., Weber C., Meder B., Hajjar R., Landmesser U. 2018. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 39 (29), 2704–2716. https://doi.org/10.1093/eurheartj/ehx165
Adams V. 2019. Assessment of micro ribonucleic acids after exercise: Is this the future to detect coronary artery disease at its early stage? Eur. J. Prev. Cardiol. 26 (4), 346–347. https://doi.org/10.1177/2047487318811958
Zou L., Ma X., Lin S., Wu B., Chen Y., Peng C. 2019 Long noncoding RNA-MEG3 contributes to myocardial ischemia-reperfusion injury through suppression of MIR-7-5p expression. Biosci. Rep. 39 (8), BSR20190210. https://doi.org/10.1042/BSR20190210
Piccoli M.T., Gupta S.K., Viereck J., Foinquinos A., Samolovac S., Kramer F.L., Garg A., Remke J., Zimmer K., Batkai S., Thum T. 2017. Inhibition of the cardiac fibroblast-enriched lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction. Circ. Res. 121 (5), 575–583. https://doi.org/10.1161/CIRCRESAHA.117.310624
Zhang J., Liang Y., Huang X., Guo X., Liu Y., Zhong J., Yuan J. 2019. STAT3-induced upregulation of lncRNA MEG3 regulates the growth of cardiac hypertrophy through miR-361-5p/HDAC9 axis. Sci. Rep. 9 (1), 460. https://doi.org/10.1038/s41598-018-36369-1
Wu Z., He Y., Li D., Fang X., Shang T., Zhang H., Zheng X. 2017. Long noncoding RNA MEG3 suppressed endothelial cell proliferation and migration through regulating miR-21. Am. J. Transl. Res. 9 (7), 3326–3335.
Li F.P., Lin D.Q., Gao L.Y. 2018. LncRNA TUG1 promotes proliferation of vascular smooth muscle cell and atherosclerosis through regulating miRNA-21/PTEN axis. Eur. Rev. Med. Pharmacol. Sci. 22 (21), 7439–7447. https://doi.org/10.26355/eurrev-201811-16284
Guo Y., Sun Z., Chen M., Lun J. 2021. LncRNA TUG1 regulates proliferation of cardiac fibroblast via the miR-29b-3p/TGF-β1 axis. Front. Cardiovasc. Med. 8, 646806. https://doi.org/10.3389/fcvm.2021.646806
Zhang G., Ni X. 2021. Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/ MEF2C axis. Open Life Sci. 16 (1), 242–251. https://doi.org/10.1515/biol-2021-0025
Foulds C.E., Tsimelzon A., Long W., Le A., Tsai S.Y., Tsai M.J., O’Malley B.W. 2010. Research resource: Expression profiling reveals unexpected targets and functions of the human steroid receptor RNA activator (SRA) gene. Mol. Endocrinol. 24 (5), 1090–1105. https://doi.org/10.1210/me.2009-0427
Ren S., Zhang Y., Li B., Bu K., Wu L., Lu Y., Lu Y., Qiu Y. 2019. Downregulation of lncRNA‑SRA participates in the development of cardiovascular disease in type II diabetic patients. Exp. Ther. Med. 17 (5), 3367–3372. https://doi.org/10.3892/etm.2019.7362
Yang S., Sun J. 2018. LncRNA SRA deregulation contributes to the development of atherosclerosis by causing dysfunction of endothelial cells through repressing the expression of adipose triglyceride lipase. Mol. Med. Rep. 18 (6), 5207–5214. https://doi.org/10.3892/mmr.2018.9497
Huang Z., Shi J., Gao Y., Cui C., Zhang S., Li J., Zhou Y., Cui Q. 2019. HMDD v3.0: A database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 47 (D1), D1013–D1017. https://doi.org/10.1093/nar/gky1010
Huang H.Y., Lin Y.C., Li J., Huang K.Y., Shrestha S., Hong H.C., Tang Y., Chen Y.G., Jin C.N., Yu Y., Xu J.T., Li Y.M., Cai X.X., Zhou Z.Y., Chen X.H., Pei Y.Y., Hu L., Su J.J., Cui S.D., Wang F., Xie Y.Y., Ding S.Y., Luo M.F., Chou C.H., Chang N.W., Chen K.W., Cheng Y.H., Wan X.H., Hsu W.L., Lee T.Y., Wei F.X., Huang H.D. 2020. MiRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 48 (D1), D148–D154. https://doi.org/10.1093/nar/gkz896
Chang L., Zhou G., Soufan O., Xia J. 2020. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 48 (W1), W244–W251. https://doi.org/10.1093/nar/gkaa467
Metsalu T., Vilo J. 2015. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 43 (W1), W566–W570. https://doi.org/10.1093/nar/gkv468
Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. 2003. Cytoscape: A software environment for integrated models. Genome Res. 13 (11), 2498‒2504. https://doi.org/10.1101/gr.1239303
Livak K.J., Schmittgen T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCΤ method. Methods. 25 (4), 402–408. https://doi.org/10.1006/meth.2001.1262
Saygili H., Bozgeyik I., Yumrutas O., Akturk E., Bagis H. 2021. Differential expression of long noncoding RNAs in patients with coronary artery disease. Mol. Syndromol. 12 (6), 372–378. https://doi.org/10.1159/000517077
Bai Y., Zhang Q., Su Y., Pu Z., Li K. 2019. Modulation of the proliferation/apoptosis balance of vascular smooth muscle cells in atherosclerosis by lncRNA-MEG3 via regulation of miR-26a/smad1 axis. Int. Heart J. 60 (2), 444–450. https://doi.org/10.1536/IHJ.18-195
Wu H., Zhao Z.A., Liu J., Hao K., Yu Y., Han X., Li J., Wang Y., Lei W., Dong N., Shen Z., Hu S. 2018. Long noncoding RNA Meg3 regulates cardiomyocyte apoptosis in myocardial infarction. Gene Ther. 25 (8), 511–523. https://doi.org/10.1038/s41434-018-0045-4
Su Q., Liu Y., Lv X.W., Dai R.X., Yang X.H., Kong B.H. 2020. LncRNA TUG1 mediates ischemic myocardial injury by targeting miR-132-3p/HDAC3 axis. Am. J. Physiol. Heart Circ. Physiol. 318 (2), H332–H344. https://doi.org/10.1152/ajpheart.00444.2019
Yan H.Y., Bu S.Z., Zhou W.B., Mai Y.F. 2018. TUG1 promotes diabetic atherosclerosis by regulating proliferation of endothelial cells via Wnt pathway. Eur. Rev. Med. Pharmacol. Sci. 22 (20), 6922–6929.
Kumar D., Narang R., Sreenivas V., Rastogi V., Bhati-a J., Saluja D., Srivastava K. 2020. Circulatory miR-133b and miR-21 as novel biomarkers in early prediction and diagnosis of coronary artery disease. Genes (Basel) . 11 (2), 164. https://doi.org/10.3390/genes11020164
Ren J., Zhang J., Xu N., Han G., Geng Q., Song J., Li S., Zhao J., Chen H. 2013. Signature of circulating MicroRNAs As potential biomarkers in vulnerable coronary artery disease. PLoS One. 8 (12), e80738. https://doi.org/10.1371/journal.pone.0080738
Fleissner F., Jazbutyte V., Fiedler J., Gupta S.K., Yin X., Xu Q., Galuppo P., Kneitz S., Mayr M., Ertl G., Bauersachs J., Thum T. 2010. Short communication: Asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ. Res. 107 (1), 138–143. https://doi.org/10.1161/CIRCRESAHA.110.216770
Weber M., Baker M.B., Moore J.P., Searles C.D. 2010. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem. Biophys. Res. Commun, 393 (4), 643–648. https://doi.org/10.1016/j.bbrc.2010.02.045
Abdallah H.Y., Hassan R., Fareed A., Abdelgawad M., Mostafa S.A., Mohammed E.A.-M. 2022. Identification of a circulating microRNAs biomarker panel for non-invasive diagnosis of coronary artery disease: Case–control study. BMC Cardiovasc. Disord. 22 (1), 286. https://doi.org/10.1186/s12872-022-02711-9
Mohammed A., Shaker O.G., Khalil M.A.F., Gomaa M., Fathy S.A., Abu-El-Azayem A.K., Samy A., Aboelnor M.I., Gomaa M.S., Zaki O.M., Erfan R. 2022. Long non-coding RNA NBAT1, TUG1, miRNA-335, and miRN-A-21 as potential biomarkers for acute ischemic stroke and their possible correlation to thyroid hormones. Front Mol. Biosci. 9, 914506. https://doi.org/10.3389/fmolb.2022.914506
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Hoda Y. Abdallah, Amr E. Ahmed and Mai Abdelgawad contributed to the study conception and design. Clinical diagnosis and interviews to participants done by Ahmed Fareed. Experimental work, material preparation, and data collection carried out by Mai Abdelgawad and Hoda Y. Abdallah. Mai Abdelgawad performed statistical and bioinformatic analyses. All authors analyzed and interpreted the patient data. All authors discussed the final results. Amr E. Ahmed and Hoda Y. Abdallah supervised the work. The first draft of the manuscript was written by Mai Abdelgawad. While Hoda Y. Abdallah, Ahmed Fareed, and Amr E. Ahmed edited and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The current study has been conformed with all institutional policies, and national rules, and was authorized by Suez Canal University Hospital (SCUH) Institutional Ethical Committee, Faculty of Medicine at Suez Canal University in Ismailia, Egypt prior to the start of the study. The study was conducted following the Declaration of Helsinki. The enrolled subjects in this study were recruited from the Suez Canal University Hospital with ethical consent number (4504). Prior to this study, both CAD cases and healthy volunteers who submitted their blood samples signed a written informed permission form, which displayed patient consent to interviews, sample collection, data access, and test result access.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Abdelgawad, M., Abdallah, H.Y., Fareed, A. et al. Long Noncoding RNAs MEG3, TUG1, and hsa-miR-21-3p Are Potential Diagnostic Biomarkers for Coronary Artery Disease. Mol Biol 57, 1186–1198 (2023). https://doi.org/10.1134/S0026893324010126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893324010126