Abstract
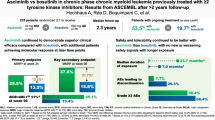
Bosutinib (BOSULIF®), an orally administered BCR–ABL tyrosine kinase inhibitor (TKI) developed by Pfizer Inc., is well established in the EU and the USA as a treatment for adults with newly diagnosed (ND) chronic phase (CP) Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML), and for CP, accelerated phase and blast phase Ph+ CML that is resistant or intolerant (R/I) to prior therapy. In September 2023, based on clinical data from patients aged ≥ 1 to < 18 years, bosutinib was approved in the USA for the treatment of pediatric patients aged ≥ 1 year with CP Ph+ CML that is ND or R/I to prior therapy. This article summarizes the milestones in the development of bosutinib leading to this first pediatric approval.
Similar content being viewed by others
Change history
18 December 2023
The original online version of this article was revised to remove the query text from the Abstract.
References
Shima H, Shimada H. Recent progress in the management of pediatric chronic myeloid leukemia. Int J Hematol. 2023;117(2):182–7.
Phillips LN, Hijiya N. Tyrosine kinase inhibitors and beyond for chronic myeloid leukemia in children. Paediatr Drugs. 2021;23(3):241–51.
Syed YY, McCormack PL, Plosker GL. Bosutinib: a review of its use in patients with Philadelphia chromosome-positive chronic myelogenous leukemia. BioDrugs. 2014;28(1):107–20.
Pfizer Inc. BOSULIF® (bosutinib) tablets and capsules, for oral use: US prescribing information. 2023. https://www.fda.gov/. Accessed 13 Oct 2023.
Pfizer Inc. BOSULIF® (bosutinib) tablets, for oral use: US prescribing information. 2012. https://www.fda.gov/. Accessed 18 Oct 2023.
European Medicines Agency. Bosulif (international non-proprietary name bosutinib): EU assessment report. 2013. https://www.ema.europa.eu/en/medicines/human/EPAR/bosulif. Accessed 18 Oct 2023.
Pfizer Inc. BOSULIF® (bosutinib) tablets, for oral use: US prescribing information. 2017. https://www.fda.gov/. Accessed 18 Oct 2023.
European Medicines Agency. Bosulif (international non-proprietary name bosutinib): EU assessment report. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/bosulif. Accessed 18 Oct 2023.
US Food & Drug Administration. FDA approves bosutinib for pediatric patients with chronic myelogenous leukemia [media release]. 26 Sep 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-bosutinib-pediatric-patients-chronic-myelogenous-leukemia.
Tauer JT, Hofbauer LC, Jung R, et al. Micro-osmotic pumps for continuous release of the tyrosine kinase inhibitor bosutinib in juvenile rats and its impact on bone growth. Med Sci Monit Basic Res. 2013;19:274–8.
Pennesi E, Brivio E, Willemse M. A phase I/II study of bosutinib in pediatric patients with resistant/intolerant or newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia, Study ITCC (Innovative Therapies for Children with Cancer European Consortium) 054 and COG (Children’s Oncology Group Consortium) AAML1921: results from the phase I trial in resistant/intolerant patients [abstract no. 2558]. Blood. 2021;138(Suppl 1):2558–60.
US National Institutes of Health. ClinicalTrials.gov identifier NCT04258943. 2023. https://clinicaltrials.gov/study/NCT04258943. Accessed 30 Oct 2023.
Keino D, Tanizawa A, Watanabe A, et al. Analysis of bosutinib for chronic myeloid leukemia in the multicenter, prospective observational study of pediatric chronic myeloid leukemia (PLSG CML-08) [abstract no. EP089/#1480]. Pediatr Blood Cancer. 2022;69(Suppl 5):S189–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of Interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sheridan M. Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoy, S.M. Bosutinib: Pediatric First Approval. Pediatr Drugs 26, 209–214 (2024). https://doi.org/10.1007/s40272-023-00608-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00608-4