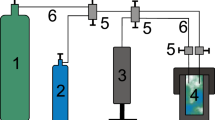
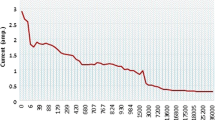
High-temperature electrochemical synthesis (HTES) in molten salts is highly promising among the up-to-date methods for the production of carbide powders. Ultrafine composite powders of tungsten carbides (WC|C, WC|C|Pt, W2C|WC, and W2C|W) were synthesized using the HTES method in electrolytic baths with different chemical compositions under various synthesis conditions (cathode current density, CO2 pressure in the electrolyzer, temperature, and cathode material). Composite powders (up to 3 wt.% free carbon) with a WC particle size of 20–30 nm were prepared using Na, K|Cl (1 : 1)–Na2W2O7 (6.4 wt.%)–CO2 (1.25 MPa) and Na, K|Cl (1 : 1)–Na2WO4 (12.0 wt.%)–NaPO3 (0.7 wt. %)–CO2 (1.25 MPa) electrolytic baths at a temperature of 750°C. When the CO2 pressure was reduced to 0.75 MPa, composite W2C|WC powders formed at the cathode. The ratio of carbide phases in the composites depended on the initial concentration of tungsten salts in the electrolyte and on the CO2 gas pressure in the electrolyzer. The addition of Li2CO3 (4.5 wt.%) to the electrolytic salt mixture decreased the tungsten carbide particles to 10 nm, changed their morphology, and increased the free carbon content in the composite up to 5 wt.%. The specific surface area of the powder increased by a factor of 4 to 7 (from 20–35 to 140 m2/g). The resulting products were modified with fine platinum particles through the use of platinum cathodes. The HTES method demonstrated its potential for producing tungsten carbide powders with the properties allowing their use as electrocatalysts in the hydrogen evolution reaction. For the WC|C composite powders synthesized in the Na, K|Cl–Na2W2O7–Li2CO3–CO2 system, the hydrogen evolution potential was –0.02 V relative to the normal hydrogen electrode, the overpotential η at a current density of 10 mA/cm2 was –110 mV, the exchange current was 7.0 ⋅ 10–4 A/cm2, and the Tafel slope was –85 mV/dec.







Similar content being viewed by others
References
I. Konyashin, “Cemented carbides for mining, construction and wear parts,” Compr. Hard Mater., 1, 425–451 (2014).
J. Sun, J. Zhao, Zh. Huang, K. Yan, X. Shen, J. Xing, Y. Gao, Y. Jian, H. Yang, and B. Li, “A review on binderless tungsten carbide: development and application,” Nano-Micro Lett., 12, Issue 13, 1–37 (2020).
D. Göhl, A.M. Mingers, S. Geiger, M. Schalenbach, S. Cherevko, J. Knossalla, D. Jalalpoor, F. Schüth, K.J.J. Mayrhofer, and M. Ledendecker, “Electrochemical stability of hexagonal tungsten carbide in the potential window of fuel cells and water electrolyzers investigated in a half-cell configuration,” Electrochim. Acta, 270, 70–76 (2018).
S. Emin, C. Altinkaya, A. Semerci, H. Okuyucu, A. Yildiz, and P. Stefanov, “Tungsten carbide electrocatalysts prepared from metallic tungsten nanoparticles for efficient hydrogen evolution,” Appl. Catal. B: Environ., 236, 147–153 (2018).
C.H. Kim, Y.G. Hur, S.H. Lee, and K.Y. Lee, “Hydrocracking of vacuum residue using nano-dispersed tungsten carbide catalyst,” Fuel, 233, 200–206 (2018).
W. Mounfield, A. Harale, and Y. Román-Leshkov, “Impact of morphological effects on the activity and stability of tungsten carbide catalysts for dry methane reforming,” Energy Fuels, 33, Issue 6, 5544–5550 (2019).
S. Ananthaneni, Z. Smith, and R.B. Rankin, “Graphene supported tungsten carbide as catalyst for electrochemical reduction of CO2,” Catalysts, 9, Issue 7, 604 (2019).
P. Bretzler, K. Köhler, A.V. Nikiforov, E. Christensen, R.W. Berg, and N.J. Bjerrum, “Efficient water splitting electrolysis on a platinum-free tungsten carbide electrocatalyst in molten CsH2PO4 at 350–390°C,” Int. J. Hydrogen Energy, 45, Issue 41, 21262–21272 (2020).
X. Xie, L. Liu, S. Chen, Y. Zhou, and X. Hu, “Investigation of commercial tungsten carbide as an HER electrocatalyst in PEMWE,” Int. J. Electrochem. Sci., 15, 3980–3995 (2020).
A. Šestan, J. Zavašnik, M.M. Kržmance, M. Kocen, P. Jenuš, S. Novak, M. Čeh, and G. Dehm, “Tungsten carbide as a deoxidation agent for plasma-facing tungsten-based materials,” J. Nucl. Mater., 524, 135–140 (2019).
N. Han, K. Liu, X. Zhang, M. Wang, P. Du, Z. Huang, D. Zhou, Q. Zhang, T. Gao, Y. Jia, L. Luo, J. Wang, and X. Sun, “Highly efficient and stable solar-powered desalination by tungsten carbide nanoarray film with sandwich wettability,” Sci. Bull., 64, Issue 6, 391–399 (2019).
N.J. AbuAlRoos, M.N. Azman, N.A.B. Amin, and R. Zainon, “Tungsten-based material as promising new lead-free gamma radiation shielding material in nuclear medicine,” Phys. Med.: Eur. J. Med. Phys., 78, 48–57 (2020).
Y.-C. Wu, Y. Yang, X.-Y. Tan, L. Luo, X. Zan, X.-Y. Zhu, Q. Xu, and J.-G. Cheng, “Preparation technology of ultra-fine tungsten carbide powder: an overview,” Front. Mater., 7, 1–11 (2020).
R. Yang, T. Xing, R. Xu, and M. Li, “Molten salt synthesis of tungsten carbide powder using a mechanically activated powder,” Int. J. Refract. Met. Hard Mater., 29, Issue 1, 138–140 (2011).
C. He, H. Meng, X. Yao, and P.K. Shen, “Rapid formation of nanoscale tungsten carbide on graphitized carbon for electrocatalysis,” Int. J. Hydrogen Energy, 37, Issue 10, 8154–8160 (2012).
P. Ranjan, T. Kurosaki, H. Suematsu, R. Jayaganthan, and R. Sarathi, “Formation of tungsten carbide nanoparticles by wire explosion process,” Int. J. Appl. Ceram. Technol., 17, Issue 1, 304–310 (2020).
B. Sankar, M. Kamaraj, S. Chakravarthy, and R. Sarathi, “Synthesis and characterization of hexagonal nano tungsten carbide powder using multi walled carbon nanotubes,” Int. J. Refract. Met. Hard Mater., 33, 53–57 (2012).
T. Dash and B.B. Nayak, “Preparation of WC–W2C composites by arc plasma melting and their characterizations,” Ceram. Int., 39, Issue 3, 3279–3292 (2013).
Z. Yan, M. Cai, and P. Shen, “Nanosized tungsten carbide synthesized by a novel route at low temperature for high performance electrocatalysis,” Sci. Rep., 3, Issue 1646, 1–7 (2013).
V.N. Krasil’nikov, E.V. Polyakov, N.A. Khlebnikov, N.V. Tarakina, and M.V. Kuznetsov, “Precursor synthesis and properties of nanodispersed tungsten carbide and nanocomposites WC:nC,” Ceram. Int., 43, Issue 5, 4131–4138 (2017).
T. Shigeru, I.A. Bataev, O. Hayato, and H. Kazuyuki, “Synthesis of metastable cubic tungsten carbides by electrical explosion of tungsten wire in liquid paraffin,” Adv. Powder Technol., 29, 2447–2455 (2018).
Y. Wu, J. Dang, Z. Lv, and R. Zhang, “The preparation of tungsten carbides and tungsten powders by reaction of tungsten trioxide with methanol,” Int. J. Refract. Met. Hard Mater., 76, 99–107 (2018).
I.A. Novoselova, E.P. Nakoneshnaya, N.A. Karpushin, V.N. Bykov, G.I. Dovbeshko, and A.D. Rynder, “Electrochemical synthesis of composites based on nanosized tungsten carbide powders from salt melts,” Metallofiz. Noveish. Tekhnol., 36, No. 4, 491–508 (2014).
I.A. Novoselova, S.V. Kuleshov, E.N. Fedoryshena, M.A. Karpushin, and V.M. Bykov, “Electrochemical synthesis of tungsten carbides in salt melts for electrocatalysis,” Ukr. Khim. Zh., 82, No. 11, 67–76 (2016).
I.A. Novoselova, S.V. Kuleshov, E.N. Fedoryshena, and V.N. Bykov, “Electrochemical synthesis of tungsten carbide in molten salts, its properties and applications,” ECS Trans., 86, Issue 14, 81–94 (2018).
I.A. Novoselova, S.V. Kuleshov, A.O. Omelchuk, V.M. Bykov, and O.M. Fesenko, “Electroreduction of ditungstate and carbonate anions in a chloride melt,” Ukr. Khim. Zh., 87, No. 12, 97–108 (2021).
I.A. Novoselova, S.V. Kuleshov, A.O. Omelchuk, V.M. Bykov, and O.M. Fesenko, “Electroreduction of Li2CO3 in an equimolar melts of sodium and potassium chlorides,” Ukr. Khim. Zh., 87, No. 6, 70–81 (2021).
I.A. Novoselova, S.V. Kuleshov, S.V. Volkov, and V.N. Bykov, “Electrochemical synthesis, morphological and structural characteristics of carbon nanomaterials produced in molten salts,” Electrochim. Acta, 211, 343–355 (2016).
L.G. Cançado, K. Takai, and T. Enoki, “General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy,” Appl. Phys. Lett., 88, Issue 16, 163106 (2006).
I.A. Novoselova, S.V. Kuleshov, A.O. Omelchuk, R.M. Savchuk, and V.M. Bykov, “Thermal stability of electrolytic nanocrystalline tungsten carbide,” Ukr. Khim. Zh., 84, No. 3, 62–68 (2018).
C.F. Holder and R.E. Schaak, “Tutorial on powder X-ray diffraction for characterizing nanoscale materials,” ACS Nano, 13, Issue 7, 7359–7365 (2019).
W. Zhou, R. Apkarian, Z.L. Wang, and D. Joy, “Fundamentals of scanning electron microscopy (SEM),” Scanning Microscopy Nanotechnology, Springer, New York (2006), pp. 1–40.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 62, Nos. 3–4 (550), pp. 14–27, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Novoselova, I.A., Kuleshov, S.V., Omelchuk, A.O. et al. Effect of Electrochemical Synthesis Conditions on the Composition, Structure, and Morphology of Tungsten Carbide Powders. Powder Metall Met Ceram 62, 142–152 (2023). https://doi.org/10.1007/s11106-023-00378-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-023-00378-1