Abstract
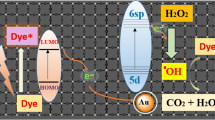
The possibility of stabilization of zinc(II) 2,3,9,10,16,17,23,24-octa[(3,5-sodium biscarboxylate)phenoxy] phthalocyaninate (ZnPc16) by its hybridization with the surface of graphene oxide (GO) sheets via van der Waals or coordination bonds with functional groups of the carbon matrix in the GO hydrosols has been investigated. A combination of physicochemical analysis methods (scanning electron microscopy, fluorescence microscopy, powder X-ray diffraction, and Raman spectroscopy) has been employed to confirm the integration of ZnPc16 with GO nanosheets and to study the morphology and structure of the obtained hybrid materials. Using electronic absorption spectroscopy, it has been found that, regardless of the hybridization method, the binding of the macrocycles to the inorganic particles increases the stability of ZnPc16 in an aqueous medium being irradiated with visible light. The analysis of spectral kinetic data has shown that, in contrast to the system obtained by direct integration of ZnPc16 and GO, the hybrid material formed by coordination bonding of the components via zinc acetate (Zn(OAc)2) as a binding metal cluster is able to exhibit photocatalytic properties in oxidative photodegradation of some model organic pollutant substrates (rhodamine 6G, 1,5-dihydroxynaphthalene, and 1,4-nitrophenol). The proposed colloid-chemical approach to the stabilization of photoactive water-soluble phthalocyaninates makes it possible to increase their resistance to photoinduced self-oxidation and can be adapted for various derivatives of tetrapyrrole compounds possessing photosensitizing properties.














Similar content being viewed by others
REFERENCES
Gyamfi, B.A., Onifade, S.T., Nwani, C., and Bekun, F.V., Accounting for the combined impacts of natural resources rent, income level, and energy consumption on environmental quality of G7 economies: A panel quantile regression approach, Environ. Sci. Pollut. Res., 2022, vol. 29, no. 2, pp. 2806–2818. https://doi.org/10.1007/s11356-021-15756-8
Ebhota, W.S. and Jen, T.C., Fossil fuels environmental challenges and the role of solar photovoltaic technology advances in fast tracking hybrid renewable energy system, International Journal of Precision Engineering and Manufacturing - Green Technology, 2020, vol. 7, no. 1, pp. 97–117. https://doi.org/10.1007/s40684-019-00101-9
Mazzeo, A., Santalla, S., Gaviglio, C., Doctorovich, F., and Pellegrino, J., Recent progress in homogeneous light-driven hydrogen evolution using first-row transition metal catalysts, Inorg. Chim. Acta, 2020, vol. 517, p. 119950. https://doi.org/10.1016/j.ica.2020.119950
Whittemore, T.J., Xue, C., Huang, J., Gallucci, J.C., and Turro, C., Single-chromophore single-molecule photocatalyst for the production of dihydrogen using low-energy light, Nat. Chem., 2020, vol. 12, no. 2, pp. 180–185. https://doi.org/10.1038/s41557-019-0397-4
Zhang, Y., Ren, K., Wang, L.L., and Fan, Z., Porphyrin-based heterogeneous photocatalysts for solar energy conversion, Chin. Chem. Lett., 2022, vol. 33, no. 1, pp. 33–60. https://doi.org/10.1016/j.cclet.2021.06.013
Liu, M.L., Guo, J.L., Japip, S., et al., One-step enhancement of solvent transport, stability and photocatalytic properties of graphene oxide/polyimide membranes with multifunctional cross-linkers, J. Mater. Chem. A, 2019, vol. 7, no. 7, pp. 3170–3178. https://doi.org/10.1039/C8TA11372F
Liu, X., Chen, Q., Lv, L., Feng, X., and Meng, X., Preparation of transparent PVA/TiO2 nanocomposite films with enhanced visible-light photocatalytic activity, Catal. Commun., 2015, vol. 58, pp. 30–33. https://doi.org/10.1016/j.catcom.2014.08.032
Das, P., Chakraborty, K., Chakrabarty, S., and Ghosh, S., Reduced graphene oxide - zinc phthalocyanine composites as fascinating material for optoelectronic and photocatalytic applications, Chemistry Select, 2017, vol. 2, no. 11, pp. 3297–3305. https://doi.org/10.1002/slct.201700384
Zhang, Z., Wang, J., Liu, D., et al., Highly efficient organic photocatalyst with full visible light spectrum through π−π stacking of TCNQ-PTCDI, ACS Appl. Mater. Interfaces, 2016, vol. 8, no. 44, pp. 30225–30231. https://doi.org/10.1021/acsami.6b10186
Mak, C.H., Han, X., Du, M., et al., Heterogenization of homogeneous photocatalysts utilizing synthetic and natural support materials, J. Mater. Chem. A, 2021, vol. 9, no. 8, pp. 4454–4504. https://doi.org/10.1039/D0TA08334H
Anaya-Rodríguez, F., Durán-Álvarez, J.C., Drisya, K.T., and Zanella, R., The challenges of integrating the principles of green chemistry and green engineering to heterogeneous photocatalysis to treat water and produce green H2, Catalysts, 2023, vol. 13, no. 1, p. 154. https://doi.org/10.3390/catal13010154
Xu, C., Ravi Anusuyadevi, P., Aymonier, C., Luque, R., and Marre, S., Nanostructured materials for photocatalysis, Chem. Soc. Rev., 2019, vol. 48, no. 14, pp. 3868–3902. https://doi.org/10.1039/C9CS00102F
Liu, Y., Tian, J., Wei, L., et al., Modified g-C3N4/TiO2/CdS ternary heterojunction nanocomposite as highly visible light active photocatalyst originated from CdS as the electron source of TiO2 to accelerate Z-type heterojunction, Sep. Purif. Technol., 2021, vol. 257, p. 117976. https://doi.org/10.1016/j.seppur.2020.117976
Zhang, X.Y., Li, H.P., Cui, X.L., and Lin, Y., Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting, J. Mater. Chem., 2010, vol. 20, no. 14, pp. 2801–2806. https://doi.org/10.1039/B917240H
Nemiwal, M., Zhang, T.C., and Kumar, D., Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity, Sci. Total Environ., 2021, vol. 767, p. 144896. https://doi.org/10.1016/j.scitotenv.2020.144896
Hsu, H.C., Shown, I., Wei, H.Y., et al., Graphene oxide as a promising photocatalyst for CO2 to methanol conversion, Nanoscale, 2013, vol. 5, no. 1, pp. 262–268. https://doi.org/10.1039/C2NR31718D
Zhao, Y.S. and Yao, J., Organic one-dimensional nanostructures: Construction and optoelectronic properties, One-Dimens. Nanostruct.: Princ. Appl., 2013, vol. 43, no. 3, pp. 381–395. https://doi.org/10.1002/9781118310342.ch17
Chen, Y.Z., Li, W.H., Li, L., and Wang, L.N., Progress in organic photocatalysts, Rare Met., 2018, vol. 37, no. 1, pp. 1–12. https://doi.org/10.1007/s12598-017-0953-2
Kosco, J., Moruzzi, F., Willner, B., and McCulloch, I., Photocatalysts based on organic semiconductors with tunable energy levels for solar fuel applications, Adv. Energy Mater., 2020, vol. 10, no. 39, p. 2001935. https://doi.org/10.1002/aenm.202001935
Díaz, U., Brunel, D., and Corma, A., Catalysis using multifunctional organosiliceous hybrid materials, Chem. Soc. Rev., 2013, vol. 42, no. 9, pp. 4083–4097. https://doi.org/10.1039/C2CS35385G
Arslanov, V.V., Kalinina, M.A., Ermakova, E.V., Raitman, O.A., Gorbunova, Y.G., et al., Hybrid materials based on graphene derivatives and porphyrin metal-organic frameworks, Russ. Chem. Rev., 2019, vol. 88, no. 8, pp. 775–799. https://doi.org/10.1070/RCR4878
Zhao, G., Pang, H., Liu, G., et al., Co-porphyrin/carbon nitride hybrids for improved photocatalytic CO2 reduction under visible light, Appl. Catal., B, 2017, vol. 200, pp. 141–149. https://doi.org/10.1016/j.apcatb.2016.06.074
Nugmanova, A.G. and Kalinina, M.A., Supramolecular self-assembly of hybrid colloidal systems, Colloid J., 2022, vol. 84, no. 5, pp. 642−662. https://doi.org/10.1134/S1061933X22700107
Gacka, E., Burdzinski, G., Marciniak, B., Kubas, A., and Lewandowska-Andralojc, A., Interaction of light with a non-covalent zinc porphyrin–graphene oxide nanohybrid, Phys. Chem. Chem. Phys., 2020, vol. 22, no. 24, pp. 13456–13466. https://doi.org/10.1039/D0CP02545C
Sorokin, A.B., Phthalocyanine metal complexes in catalysis, Chem. Rev., 2013, vol. 113, no. 10, pp. 8152–8191. https://doi.org/10.1021/cr4000072
Jin, L., Lv, S., Miao, Y., Liu, D., and Song, F., Recent development of porous porphyrin-based nanomaterials for photocatalysis, ChemCatChem, 2021, vol. 13, no. 1, pp. 140–152. https://doi.org/10.1002/cctc.202001179
Liu, W., Jensen, T.J., Fronczek, F.R., et al., Synthesis and cellular studies of nonaggregated water-soluble phthalocyanines, J. Med. Chem., 2005, vol. 48, no. 4, pp. 1033–1041. https://doi.org/10.1021/jm049375b
Gregory, P., Industrial applications of phthalocyanines, J. Porphyrins Phthalocyanines, 2000, vol. 4, no. 4, pp. 432–437. https://doi.org/10.1002/(SICI)1099-1409(200006/07)4:4<432::AID-JPP254>3.0.CO;2-N
Nikoloudakis, E., López-Duarte, I., Charalambi-dis, G., Ladomenou, K., Ince, M., and Coutso-lelos, A.G., Porphyrins and phthalocyanines as biomimetic tools for photocatalytic H2 production and CO2 reduction, Chem. Soc. Rev., 2022, vol. 51, no. 16, pp. 6965–7045. https://doi.org/10.1039/D2CS00183G
Liu, Y., Zuo, P., Wang, F., et al., Covalent immobilization of phthalocyanine on graphene oxide for the degradation of phenol, J. Taiwan Inst. Chem. Eng., 2019, vol. 104, pp. 187–200. https://doi.org/10.1016/j.jtice.2019.09.007
Qian, J., Liu, Y., Zheng, W., Zhou, B., and Dong, X., Covalent modification of iron phthalocyanine into skeleton of graphitic carbon nitride and its visible-light-driven photocatalytic reduction of nitroaromatic compounds, Catalysts, 2022, vol. 12, no. 7, p. 752. https://doi.org/10.3390/catal12070752
Jiang, B.P., Hu, L.F., Wang, D.J., et al., Graphene loading water-soluble phthalocyanine for dual-modality photothermal/photodynamic therapy via a one-step method, J. Mater. Chem. B, 2014, vol. 2, no. 41, pp. 7141–7148. https://doi.org/10.1039/C4TB01038H
Kumar, P., Kumar, A., Sreedhar, B., et al., Cobalt phthalocyanine immobilized on graphene oxide: An efficient visible-active catalyst for the photoreduction of carbon dioxide, Chem.-Eur. J., 2014, vol. 20, no. 20, pp. 6154–6161. https://doi.org/10.1002/chem.201304189
Dyrda, G., Kocot, K., Poliwoda, A., et al., Hybrid TiO2 @ phthalocyanine catalysts in photooxidation of 4-nitrophenol: Effect of the matrix and sensitizer type, J. Photochem. Photobiol., A, 2020, vol. 387, p. 112124. https://doi.org/10.1016/j.jphotochem.2019.112124
Prasad, C., Liu, Q., Tang, H., et al., An overview of graphene oxide supported semiconductors based photocatalysts: Properties, synthesis and photocatalytic applications, J. Mol. Liq., 2020, vol. 297, p. 111826. https://doi.org/10.1016/j.molliq.2019.111826
Lu, K.Q., Li, Y.H., Tang, Z.R., and Xu, Y.J., Roles of graphene oxide in heterogeneous photocatalysis, ACS Mater. Au., 2021, vol. 1, no. 1, pp. 37–54. https://doi.org/10.1021/acsmaterialsau.1c00022
Motevalli, B., Fox, B.L., and Barnard, A.S., Charge-dependent Fermi level of graphene oxide nanoflakes from machine learning, Comput. Mater. Sci., 2022, vol. 211, p. 111526. https://doi.org/10.1016/j.commatsci.2022.111526
Hu, X., Mu, L., Wen, J., and Zhou, Q., Covalently synthesized graphene oxide-aptamer nanosheets for efficient visible-light photocatalysis of nucleic acids and proteins of viruses, Carbon, 2012, vol. 50, no. 8, pp. 2772–2781. https://doi.org/10.1016/j.carbon.2012.02.038
Nugmanova, A.G. and Kalinina, M.A., Self-assembly of metal-organic frameworks in pickering emulsions stabilized with graphene oxide, Colloid J., 2021, vol. 83, no. 5, pp. 614–626. https://doi.org/10.1134/S1061933X21050094
Meshkov, I.N., Zvyagina, A.I., Shiryaev, A.A., et al., Understanding self-assembly of porphyrin-based SURMOFs: How layered minerals can be useful, Langmuir, 2018, vol. 34, no. 18, pp. 5184–5192. https://doi.org/10.1021/acs.langmuir.7b04384
Nugmanova, A.G., Safonova, E.A., Baranchikov, A.E., et al., Interfacial self-assembly of porphyrin-based SURMOF/graphene oxide hybrids with tunable pore size: An approach toward size-selective ambivalent heterogeneous photocatalysts, Appl. Surf. Sci., 2022, vol. 579, p. 152080. https://doi.org/10.1016/j.apsusc.2021.152080
Sladkevich, S., Gun, J., Prikhodchenko, P.V., et al., Peroxide induced tin oxide coating of graphene oxide at room temperature and its application for lithium ion batteries, Nanotechnology, 2012, vol. 23, no. 48, p. 485601. https://doi.org/10.1088/0957-4484/23/48/485601
Vagin, S.I., Ott, A.K., and Rieger, B., Paddle-wheel zinc carboxylate clusters as building units for metal-organic frameworks, Chem. Ing. Tech., 2007, vol. 79, no. 6, p. 767. https://doi.org/10.1002/cite.200700062
Jaafar, E., Kashif, M., Sahari, S.K., et al., Study on morphological, optical and electrical properties of graphene oxide (GO) and reduced graphene oxide (rGO), Mater. Sci. Forum, 2018, vol. 917, p. 112. https://doi.org/10.4028/www.scientific.net/MSF.917.112
Gusarova, E.A., Zvyagina, A.I., Aleksandrov, A.E., et al., Interfacial self-assembly of ultrathin polydiacetylene/graphene oxide nanocomposites: A new method for synergetic enhancement of surface charge transfer without doping, Colloid Interface Sci. Commun., 2022, vol. 46, p. 100575. https://doi.org/10.1016/j.colcom.2021.100575
Zvyagina, A.I., Gusarova, E.A., Averin, A.A., et al., Structural effect of perylene derivatives on their interaction with reduced graphene oxide monolayers, Russ. J. Inorg. Chem., 2021, vol. 66, p. 273. https://doi.org/10.1134/S0036023621020224
Funding
This work was supported by the Russian Science Foundation (project no. 23-73-00095).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nugmanova, A.G., Gorshkova, A.I., Yagodin, A.V. et al. Noncovalent Stabilization of Water-Soluble Zinc Phthalocyaninate in Graphene Oxide Hydrosol. Colloid J 85, 961–974 (2023). https://doi.org/10.1134/S1061933X23600859
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X23600859