Abstract
The quasi-ternary system Cu2Se-In2Se3-CuI has been investigated by x-ray diffraction and differential thermal analysis. The isothermal section at 770 K and the liquidus surface projection of the system have been built. For the first time, the primary crystallization regions, and the coordinates of the invariant and monovariant equilibria have been determined. In the system, the regions of the solid solutions based on the binary, ternary, and quaternary compounds have been investigated. The formation of the CuIn2Se3I quaternary compound, which melts congruently at 1213 K and has a homogeneity region of 15 and 9 mol.% CuI within the composition triangle has been established. For the first time, the crystal structures of CuGa2Te3I and AgGa2Te3Br compounds have been studied using a powder method. They crystallize in the tetragonal symmetry, Space Group I-4, a = 5.9147(4) Å, c = 11.952(2) Å for CuGa2Te3I; a = 6.2977(3) Å, c = 11.9473(7) Å for AgGa2Te3Br compound, respectively. The connection of their structures with the structures of the defective diamond-like semiconductors has been discussed.
Similar content being viewed by others
1 Introduction
The multiphase compositions used in semiconductor devices require the study of phase equilibria in multicomponent systems. Therefore, the Cu2Se-In2Se3-CuI system of the mixed 2-anion chalcogen halide type has been chosen for this study. The quasi-ternary system is formed by binary halides and chalcogenides, which already have vast practical application, in particular AIYVII, where the number of cations is equal to the number of anions (AI-Cu, Ag; YVII-Cl, Br, I), cation excess compound AI2XVI, where AI-Cu, Ag; XVI-S, Se, Te, and cation-defective BIII2XVI3 compounds, where BIII-Ga, In. Since the compounds formed in this system belong to diamond-like semiconductors of the AIBIIIXVI2 and AIYVII types, it will be interesting to investigate the interaction between chalcogenides and halides. The construction of the quasi-binary phase diagrams and liquidus surface projection of the quasi-ternary system allows for determining the regions of the primary crystallization of the compounds and the coordinates of the invariant and monovariant equilibria. Previously, we partially investigated the system Cu2Se-In2Se3-CuI and established a character of the CuIn2Se3I quaternary compound formation in the In2Se3-CuI system.[1] In this work, we present additional results obtained for 3 vertical sections (Cu3InSe3-″Cu3SeI″; ″Cu3SeI″-CuIn2Se3I; CuIn3Se5-CuIn2Se3I), results for the isothermal section at 770 K and the liquidus surface projection of the quasi-ternary system Cu2Se-In2Se3-CuI. The authors of Ref 2, 3 studied quaternary compounds AIBIII2XVI3YVII, where AI-Cu, Ag; BIII-In; XVI-S, Se, Te; YVII-Cl, Br, I. Phases with structures of the defective zincblende, spinel, and defective NaCl, respectively, were obtained. For example, it was established that the CuIn2Se3I compound crystallizes in the cubic symmetry, Space Group (SG) F-43 m, a = 5.781(1) Å.[2] In our work, we decided to investigate the crystal structures of the other quaternary compounds of such type, where BIII-Ga. Some of them were investigated by us previously, like CuGa2S3I,[4] CuGa2Se3I,[4] AgGa2S3Cl,[5] AgGa2Se3Cl,[6] AgGa2Se3Br,[6] AgGa2Te3Cl,[7] AgGa2Te3I.[8] In this work, CuGa2Te3I and AgGa2Te3Br compounds were synthesized to investigate their crystal structures for a better understanding of the nature of the quaternary compounds. Their crystal structures and connection with known defective semiconductors were discussed.
2 Method of Synthesis
Simple substances of high purity (Cu-99.99, In-99.99, Se-99.997 wt.%) were used to synthesize all alloys of the investigated systems. Cuprous iodide was obtained by the interaction of CuSO4·5H2O with NaI taken in stoichiometric amounts in the presence of SO2. During the interaction of the solutions, a brown precipitate was formed, which, after passing SO2, turned into a white precipitate of cuprous iodide. The precipitate was filtered on a Buchner funnel and washed with water to remove SO42− ions. It was washed with ethanol and diethyl ether to prevent the product from oxidizing. The ampoules with prepared weights were evacuated to a residual pressure of 1.33·10−2 Pa and sealed using a gas-oxygen burner. Before the synthesis, pumped and sealed ampoules were placed in metal tubes. The synthesis was carried out in the automatic furnaces ″Thermodent″ with a furnace temperature regulation system of ± 5 K. Samples were synthesized as follows: heating to 670 K at a rate of 10 K/h, annealing for 48 h; heating to a maximum temperature of 1070 K, holding for 48 h; cooling to a temperature of 770 K at a rate of 20 K/h and homogenizing annealing was carried out for 300 h to establish the equilibrium state of the synthesized alloys.[1] They were investigated by x-ray diffraction (XRD) method on DRON 4-13 diffractometer (CuKα radiation) and differential thermal analysis (DTA) (″Thermodent″ H307/1 furnace with a PDA-1 XY-recorder, Pt/Pt-Rh thermocouple). To study the crystal structure of CuGa2Te3I and AgGa2Te3Br, high purity Cu-99.99, Ag-99.99, Ga-99.999 and Te-99.99 wt.% were used. AgBr was obtained by reacting the AgNO3 water solution with the KBr solution.
3 Results and Discussion
3.1 The Isothermal Section of the Quasi-Ternary System Cu2Se-In2Se3-CuI at 770 K (497 °C)
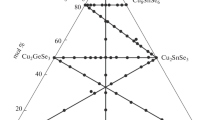
The isothermal section of the quasi-ternary system Cu2Se-In2Se3-CuI at 770 K (497 °C) was constructed based on the results of x-ray diffraction analysis (Fig. 1). According to the obtained data, the CuI compound crystallizes in cubic symmetry, SG \(Fm\overline{3}m\), a = 6.1512(3) Å, which agrees well with Ref 9 (Fig. 2). In the system In2Se3-CuI, the existence of the quaternary compound CuIn2Se3I, which crystallizes in cubic symmetry, is confirmed, SG \(F\overline{4}3m\), a = 5.8012(1) Å, which is in good agreement with Ref 2. Cu2Se is indexed as monoclinic symmetry, SG C2/c, a = 7.1379 Å, b = 12.3823 Å, c = 27.3904 Å, β = 94.308°.[10] In2Se3 is indexed as hexagonal symmetry, SG P63/mmc, with unit cell periods a = 4.0242(5) Å, c = 19.251(2) Å, which agrees well with Ref 11. The preliminary results of the x-ray phase analysis of the Cu2Se-In2Se3 system were described in our previous work.[12,13,14] The following ternary compounds were established: CuInSe2, SG \(I\overline{4}2d\), a = 5.7855(2) Å, c = 11.551(3) Å; CuIn3Se5, SG \(P\overline{4}2c\), a = 5.7602(1) Å, c = 11.515(3) Å; CuIn7Se11, SG P3m1, a = 4.0263(2) Å, c = 16.2992(7) Å; and layered CuIn5Se8 and CuIn11Se17 compounds with unknown structures. The largest single-phase regions in Cu2Se-In2Se3-CuI are based on CuInSe2 and CuIn2Se3I compounds. It is known that CuIn2Se3I is a cation defect compound, with a ratio of cations to anions of 3:4. In our opinion, this affects the largest extent of the solid solution based on CuIn2Se3I towards defective compounds CuIn3Se5, CuIn5Se8, CuIn7Se11, CuIn11Se17, but not to the CuInSe2 or CuI side, which have the same number of cations and anions. Solubility based on all other binary and ternary compounds is negligible. Between the single-phase regions there are regions of 2-phase equilibria, which divide the system into corresponding 3-phase fields.
3.2 The Liquidus Surface Projection of the Cu2Se-In2Se3-CuI Quasi-Ternary System
The liquidus surface projection (Fig. 3) was built based on the results of the DTA analyses of more than 150 samples (Fig. 4). It consists of fields of primary crystallization of α-solid solution based on HTM-Cu2Se (e2-U1-U2-p1-u (e2-U1-e3-Cu3InSe3-e2), ζ-solid solution based on HTM-CuInSe2 (e3-U1-U2-E1-m1-E2-U3-e1-U4-p4-CuInSe2-e3), ε-solid solution based on LTM-CuInSe2 (m1-E2-p2-E1-m1), η-solid solution based on HTM-CuI (p1-U2-E1-p2-E2-U3-p3-CuI-p1), δ-solid solution based on 1-HTM-In2Se3 (e4-E3-e5-In2Se3-e4), θ-solid solution based on CuIn2Se3I (e5-E3-U5-U4-e1-U3-p3-CuIn2Se3I-e5), compounds CuIn5Se8 (p4-U4-U5-p5-p4), CuIn11Se17 (p5-U5-E3-e4-p5). These areas are separated by 19 monovariant curves and 19 nonvariant points. The systems CuInSe2-CuI and CuInSe2-CuIn2Se3I are quasi-binary (Fig. 5, 6, 7, 8)[1] and divide the investigated quasi-ternary system into 3 subsystems Cu2Se-CuInSe2-CuI, CuInSe2-CuIn2Se3I-CuI and CuInSe2-CuIn2Se3I-In2Se3. To simplify the reading of the following text the formulas of the compounds and their polymorphic modifications on which the solid solutions are based will be indicated in parentheses. Three nonvariant transition reactions take place in the first subsystem (Fig. 9). The first one, LU1 + Cu3InSe3 ↔ ζ(HTM-CuInSe2) + α(HTM-Cu2Se), takes place at 1185 K (912 °C). Curves of monovariant processes: Le2-U1 ↔ Cu3InSe3 + α(HTM-Cu2Se), Le3-U1 ↔ ζ(HTM-CuInSe2) + Cu3InSe3 converge to the point U1. The second nonvariant transition reaction LU2 + α(HTM-Cu2Se) ↔ η(HTM-CuI) + ζ(HTM-CuInSe2) occurs at 1010 K (737 °C). Curves of monovariant processes converge to the point U2: LU1-U2 ↔ α(HTM-Cu2Se) + ζ(HTM-CuInSe2), Lp1-U2 ↔ η(HTM-CuI) + α(HTM-Cu2Se). Point E1 lies on the plane of the nonvariant eutectic process LE1 ↔ ε(LTM-CuInSe2) + η(HTM-CuI) + ζ(HTM-CuInSe2), which takes place at 978 K (705 °C). Curves of monovariant processes converge to this nonvariant point: LU2-E1 ↔ η(HTM-CuI) + ζ(HTM-CuInSe2), Lm1-E1 ↔ ζ(HTM-CuInSe2) + ε(LTM-CuInSe2) and Lp2-E1 ↔ η(HTM-CuI) + ε(LTM-CuInSe2). As the temperature decreases, another nonvariant eutectoid process ζ(HTM-CuInSe2) ↔ ε(LTM-CuInSe2) + η(HTM-CuI) + α(HTM-Cu2Se) occurs in the subsolidus region at 890 K (617 °C). Below it, the alloys contain crystals of 3 phases: ε(LTM-CuInSe2), η(HTM-CuI), α(HTM-Cu2Se), which agrees with Fig. 1.
Phase diagram of CuInSe2-CuI system: 1−L, 2−L + ζ, 3−L + ε, 4−L + η, 5−ζ, 6−ζ + ε, 7−ε, 8-η − ε, 9-η, with ζ and ε-solid solutions based on HTM-CuInSe2 and LTM-CuInSe2, accordingly, η-solid solution based on HTM-CuI[1]
Phase diagram of CuInSe2-CuIn2Se3I system: 1−L, 2−L + ζ, 3−L + θ, 4−ζ, 5−ζ + θ, 6−θ, 7−ζ + ε, 8−ε, 9−ε + θ, with ζ-solid solution based on HTM-CuInSe2, ε-solid solution based on LTM-CuInSe2, θ-solid solution based on CuIn2Se3I[1]
In the subsystem CuInSe2-CuIn2Se3I-CuI the nonvariant process LU3 + θ(CuIn2Se3I) ↔ η(HTM-CuI) + ζ(HTM-CuInSe2) takes place at 1000 K (727 °C). Further, crystallization is completed by the nonvariant eutectic process LE2 ↔ η(HTM-CuI) + ε(LTM-CuInSe2) + ζ(HTM-CuInSe2) at 975 K (702 °C), and in the subsolidus region at 900 К (627 °C) the eutectoid process ζ(HTM-CuInSe2) ↔ η(HTM-CuI) + ε(LTM-CuInSe2) + θ(CuIn2Se3I) takes place, and the alloys contain the corresponding 3 phases (Fig. 1). In the subsystem CuInSe2-CuIn2Se3I-In2Se3 the following nonvariant processes take place: LU4 + ζ(HTM-CuInSe2) ↔ θ(CuIn2Se3I) + CuIn5Se8 at 1123 K (850 °C); LU5 + CuIn5Se8 ↔ θ(CuIn2Se3I) + CuIn11Se17 at 1073 K (800 °C), then crystallization completes through the nonvariant eutectic process LE3 ↔ θ(CuIn2Se3I) + CuIn11Se17 + δ(1-HTM-In2Se3) at 1055 K (782 °C).
Part of the compounds in the system Cu2Se-In2Se3 are formed by solid-phase reactions, namely CuIn3Se5 and CuIn7Se11. Therefore, there are no regions of the primary crystallization on the liquidus surface projection with them. The Scheil reaction scheme representing the sequence of all invariant reactions is shown on Fig. 9.
3.3 The Vertical Section Cu3InSe3-″Cu3SeI″
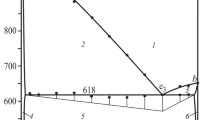
The vertical section Cu3InSe3-″Cu3SeI″ was built based on the DTA and x-ray phase analysis results. It passes through 2 surfaces of the primary crystallization of the compound Cu3InSe3 and α-solid solution, respectively (Fig. 10). The 3-phase space of secondary crystallization of the binary eutectic L ↔ α + Cu3InSe3 descends to the plane of the nonvariant process LU1 + Cu3InSe3 ↔ α + ζ, which is shown with a horizontal line at 1185 K (912 °C). There are 3-phase spaces of solid phase decomposition Cu3InSe3 ↔ α + ζ and monovariant eutectic process L ↔ ζ + α below the line at 1185 K (912 °C). The 3-phase space of the eutectic process descends to the plane of the nonvariant process LU2 + α ↔ η + ζ at 1010 K (737 °C), which results in the disappearance of the liquid. At 890 K (617 °C), this section intersects the plane of the eutectoid reaction: ζ(HTM-CuInSe2) ↔ ε(LTM-CuInSe2) + η(HTM-CuI) + α(HTM-Cu2Se), below which the alloys are 3-phase ε + η + α in agreement with Fig. 1.
The vertical section Cu3InSe3-″Cu3SeI″: 1−L; 2−L + α; 3−L + Cu3InSe3; 4−L + α + Cu3InSe3; 5−Cu3InSe3; 6−Cu3InSe3 + α; 7−Cu3InSe3 + α + ζ; 8−α + ζ; 9−L + α + ζ; 10−L + α + η; 11−L + η + ζ; 12−α + ζ + ε; 13−α + ε; 14−α + η + ε; 15−η + α, with ζ-solid solution based on HTM-CuInSe2, ε-solid solution based on LTM-CuInSe2, α-solid solution based on Cu2Se, η-solid solution based on CuI
3.4 The Vertical Section ″Cu3SeI″-CuIn2Se3I
″Cu3SeI″-CuIn2Se3I was built based on the DTA, x-ray phase analysis results and passes through 2 subsystems Cu2Se-CuInSe2-CuI and CuInSe2-CuIn2Se3I-CuI (Fig. 11). The liquidus of the section is represented by the curves of the primary crystallization of α, ζ, θ-solid solutions. In the subsystem Cu2Se-CuInSe2-CuI, the section crosses the plane of the nonvariant reaction LU2 + α ↔ η + ζ at 1010 K (737 °C), resulting in one region where the liquid disappears for some of the compositions (field 14, the 3-phase (α + η + ζ) region) while in the other region the crystals of α-solid solution disappear. Therefore, below the horizontal at 1010 K (737 °C) is field 12, where the monovariant eutectic process L ↔ η + ζ occurs. This field, together with the 3-phase field 10 (L + ε + η) of the monovariant eutectic process L ↔ ε + η, descends to the plane of nonvariant eutectic process LE1 ↔ ε + η + ζ at 978 K (705 °C). At 890 K (617 °C), this section intersects the plane of the nonvariant eutectoid reaction ζ(HTM-CuInSe2) ↔ ε(LTM-CuInSe2) + η(HTM-CuI) + α(HTM-Cu2Se). Below this plane, the alloys are 3-phase and contain crystals of ε, η, α -solid solutions (field 17), which agrees with the isothermal section in Fig. 1.
The vertical section ″Cu3SeI″-CuIn2Se3I: 1−L; 2−L + α; 3−L + ζ; 4−L + θ; 5−L + θ + ζ; 6−θ + ζ; 7−ζ + η + θ; 8−η + ε + ζ; 9−L + ζ + η; 10−L + ε + η; 11−L + ζ + α; 12−L + ζ + η; 13−ζ + η; 14−α + ζ + η; 15−L + α + η; 16−α + η; 17−α + ε + η; 18−ζ + ε + η; 19−η + ε; 20−η + ε + θ; 21−η + θ; 22−θ, with ζ-solid solution based on HTM-CuInSe2, ε-solid solution based on LTM-CuInSe2, θ-solid solution based on CuIn2Se3I, α-solid solution is based on Cu2Se, η-solid solution based on CuI.
In the subsystem CuInSe2-CuIn2Se3I-CuI at 1000 K (727 °C), the section intersects the plane of the nonvariant process LU3 + θ ↔ η + ζ, which results in the disappearance of the liquid only in a part of the alloys of the section, that is why field 7 contains 3 phases: θ + η + ζ. In the other part of the section, the nonvariant transition reaction LU3 + θ ↔ η + ζ results in the disappearance of the θ-solid solution. Therefore, below the horizontal at 1000 K (727 °C) is field 9, where the monovariant eutectic process L ↔ η + ζ takes place, which together with the 3-phase field L + ε + η of the monovariant eutectic process L ↔ ε + η descends to the plane of the nonvariant eutectic process L ↔ η + ε + ζ at 975 K (702 °C). The vertical section intersects the plane of the nonvariant eutectoid decomposition ζ(HTM-CuInSe2) ↔ η(HTM-CuI) + ε(LTM-CuInSe2) + θ(CuIn2Se3I) at 900 K (627 °C). Below this plane, the alloys are 3-phase and contain the crystals of η, ε, θ-solid solutions (field 20), which agrees with Fig. 1. The region between fields 7 and 8, is 2-phase η + ζ, since the process LU3 + θ ↔ η + ζ at 1000 K (727 °C) for the composition of 30 mol.% ″Cu3SeI″-70 mol.% CuIn2Se3I ends with the disappearance of the liquid and the crystals of θ-solid solution because this composition coincides with the connecting horizontal of the plane of the nonvariant process LU3 + θ ↔ η + ζ.
3.5 The Vertical Section CuIn3Se5-CuIn2Se3I
The section was built based on the DTA results and x-ray phase analysis. The the regions with liquid (Fig. 12) are represented by the areas of primary crystallization of ζ-solid solution based on HTM-CuInSe2 and θ-solid solution based on CuIn2Se3I. The section intersects the plane of the nonvariant process LU4 + ζ ↔ θ + CuIn5Se8 at 1123 K (850 °C) where for compositions in this section the liquid disappears. In the subsolidus region, 2 planes of nonvariant processes ζ ↔ θ + CuIn5Se8 + Cu2In4Se7 (1080 K) (807 °C), CuIn5Se8 + Cu2In4Se7 ↔ ξ + θ (1030 K) (757 °C) intersect with the section, where ξ is solid solution based on CuIn3Se5. In alloys of this section, this results in the disappearance of CuIn5Se8 and Cu2In4Se7 crystals, so below 1030 K (757 °C), crystals of ξ- and θ-solid solutions are present which agrees with Fig. 1. As the temperature decreases to 770 K (497 °C), the limit of ξ -solid solution decreases to 3 mol.% of the second component. The parameters of the unit cell increase a little from a = 5.7602(1) Å, c = 11.515(3) Å for CuIn3Se5 till a = 5.7657(2) Å, c = 11.525(4) Å for the composition of 95 mol.% CuIn3Se5-5 mol.% CuIn2Se3I. The region of θ-solid solution narrows to 17 mol.% CuIn3Se5 with a decrease in temperature to 770 K (497 °C). The parameter of the unit cell decreases from a = 5.8012(1) Å for CuIn2Se3I till a = 5.7722(3) Å for the composition of 20 mol.% CuIn3Se5-80 mol.% CuIn2Se3I.
The vertical section CuIn3Se5-CuIn2Se3I: 1−L, 2−L + ζ, 3−L + ζ + θ, 4−L + θ, 5−ζ, 6−CuIn5Se8 + ζ, 7−L + CuIn5Se8 + ζ, 8−θ + CuIn5Se8 + ζ, 9−ζ + θ, 10−θ, 11−ζ + θ, 12−Cu2In4Se7 + θ, 13−Cu2In4Se7 + ζ + θ, 14−Cu2In4Se7 + ζ, 15−Cu2In4Se7 + CuIn5Se8 + ζ, 16−CuIn5Se8 + Cu2In4Se7, 17−CuIn5Se8 + Cu2In4Se7 + θ, 18−Cu2In4Se7 + CuIn5Se8 + CuIn3Se5, 19−Cu2In4Se7 + CuIn3Se5, 20−CuIn3Se5 (ξ), 21−CuIn3Se5 + θ, with ζ-solid solution based on HTM-CuInSe2, θ-solid solution based on CuIn2Se3I
3.6 Crystal Structure of AIBIII 2XVI 3YVII Compounds, Where AI-Cu, Ag; BIII-Ga; XVI-Cl, Br, I; YVII-S, Se, Te
When the In2Se3-CuI system was investigated in Ref 1, the formation of the quaternary compound CuIn2Se3I, which belongs to a larger group of compounds with general formula AIBIII2X3Y (AI-Cu, Ag; BIII-Ga, In; X-S, Se, Te; Y-Cl, Br, I) was established. Replacing Cu+, In3+, Se2− and I− by Ag+, Ga3+, Te2−, and Br− the quaternary compounds CuGa2Te3I, AgGa2Te3Br are obtained, for which the crystal structures were studied using the powder method. The measurement conditions and the calculation results are shown in Tables 1, 2, 3, 4, Fig. 13, 14. The coordinates and the isotropic thermal parameters of atoms in the structures of the CuGa2Te3I and AgGa2Te3Br are given in Tables 2 and 3. The interatomic distances and coordination numbers of the atoms are shown in Table 4. The Ga atoms occupy 2 Wyckoff positions 2a and 2c, have tetrahedral coordination and occupy these positions to 80% (Tables 2, 3; Fig. 15). 2 Wyckoff positions (2b, 2d) are occupied by the statistical mixtures M1 (Cu (Ag) + Ga) and M2 (Cu (Ag) + Ga), resulting in coordination polyhedron-tetrahedron [M1 4Te], [M2 4Te]. The statistical mixtures M1 and M2 are 50% Cu (Ag) and 20% Ga, and 30% of positions are not occupied.
As previously mentioned, chalcohalides of the type AICIII2XVI3YVII belong to cation-deficient compounds with a ratio of cations to anions of 3:4. The structure can be represented as a 3-layer packing of anions in which ¾ of the tetrahedral vacancies are occupied by CIII cations, for example, Ga or In. Statistical mixtures of M1 (Cu(Ag) + Ga) and M2 (Cu(Ag) + Ga), and ¼ of the voids remain vacant.[15] According to Ref 16, other compounds have a cation:anion ratio of 3:4, for example, AgIn5Se8, AgZnPS4, Cu2HgI4, and Hg2SnSe4. A similar ratio have CdGa2Se4 and β-Ag2HgI4, structural type CdAl2S4, both crystallize in the SG I-4. In the specified structures of the studied compounds AICIII2XVI3YVII and CdGa2Se4, β-Ag2HgI4, a similar arrangement of cations is observed (Table 5), but in AICIII2XVI3YVII the AI atoms half occupy 2 positions 2b, 2d. The CIII cations are statistically in the same positions as AI, filling them to 20%. The CIII positions, 2a and 2c, remain partially occupied at 80%. In the compounds CdGa2Se4 and β-Ag2HgI4, position 2a is occupied by a divalent cation, and position 2c and 2b-by other cations in the structure (Ga and Ag, respectively). The vacancy occupies the 2d position in these structures.
The composition of the cation-deficient compounds with a ratio of cations to anions of 3:4 can be represented by the formula Kn-u□uAn, where K-cations, □-vacancies, A-anions, and u-the first letter of the word ″unoccupied″, which indicates the number of vacancies. For these compounds VEC > 4, in particular, AgIn5Se8, AgZnPS4, Cu2HgI4, and Hg2SnSe4, VEC = 4.571.[17, 18] The exact value is obtained for the quaternary chalcohalides AICIII2XVI3YVII (1⋅1 + 2⋅3 + 3⋅6 + 1⋅7)/1 + 2 + 3 + 1 = 4.571. If we consider the vacancy as an atom with zero valence, then we get for the compounds AICIII2□XVI3YVII 1⋅1 + 2⋅3 + 1⋅0 + 3⋅6 + 1⋅7)/1 + 2 + 1 + 3 + 1 = 4, which means that their structures are tetrahedral when the cations are surrounded by the 4 nearest anions located at the vertices of the tetrahedron.
4 Conclusions and Future Work
The quasi-ternary system Cu2Se-In2Se3-CuI formed by binary halides and chalcogenides, which already have wide practical applications, has been investigated by x-ray and differential thermal methods. The isothermal section at 770 K (497 °C) and the liquidus surface projection of the system have been built. The regions of primary crystallization, types, and coordinates of the invariant and monovariant equilibria have been established for the first time. It allows us to know the areas of primary crystallization of the compounds, types and the coordinates of the invariant and monovariant equilibria. In the system, the regions of the solid solutions based on the binary, ternary, and quaternary compounds have been investigated. The formation of the CuIn2Se3I quaternary compound, which melts congruently at 1213 K (940 °C) and has a homogeneity region of 15 and 9 mol.% CuI in the composition triangle, has been established. For the first time, the crystal structures of CuGa2ITe3 and AgGa2BrTe3 compounds, which belong to the cation-deficient compounds AICIII2XVI3YVII with a ratio of cations to anions of 3:4, have been studied using a powder method. They crystallize in the tetragonal symmetry, SG I-4, a = 5.9147(4) Å, c = 11.952(2) Å for CuGa2ITe3; a = 6.2977(3) Å, c = 11.9473(7) Å for AgGa2BrTe3 compound. The connection of their structures with the structures of the defect diamond-like semiconductors has been discussed, and a conclusion about their semiconducting properties has been made.
According to the results, future work will be on growing the single crystals of the quaternary compound CuIn2Se3I and solid solutions formed in the system to investigate their semiconducting properties.
References
V.S. Kozak, I.A. Ivashchenko, and I.D. Olekseiuk, Phase Equilibria in the Quasi-Ternary System Cu2Se-In2Se3-CuI, Uzhhorod Univ. Sci. Bull. Chem. Ser., 2019, 42, p 26-34. https://doi.org/10.24144/2414-0260.2019.2.26-34
K.-J. Range, H.J. Huebner, and B. Teil, Hochdrucksynthese Quaternärer Chalkogenidhalogenide AB2X3Y (A-Cu, Ag; B-In; X-S, Se, Te; Y-Cl, Br, I), Anorg. Chem., 1983, 38, p 155-160. https://doi.org/10.1515/znb-1983-0207
K.-J. Range and K. Handrick, New 1320637 Compounds, Z. Naturforsch., 1988, 43, p 240-242. https://doi.org/10.1515/znb-1988-0218
V.S. Kozak, P.V. Tyshchenko, I.D. Olekseiuk, I.A. Ivashchenko, and L.D. Gulay, Crystal Structure of CuGa2S(Se)3I Compounds, Odesa Univ. Sci. Bull. Chem. Ser., 2019, 72, p 63-69. https://doi.org/10.18524/2304-0947.2019.4(72).185534
P.V. Tyshchenko, Phase Equilibria of Quasi-Triple Systems Based on the Compounds of AI2X, BIII2X3, R2X3, AIY (AI-Cu, Ag; BIII-Ga, In; R-La, Er; X-S, Se; Y-Cl, I) and Properties of Intermediate Phases and Glasses. PhD thesis. 02.00.01, Uzhgorod, 2019, 154 p. https://www.uzhnu.edu.ua/uk/infocentre/get/21024
I. Ivashchenko, V. Kozak, L. Gulai, and I. Olekseiuk, Crystal Structure of AgGa2Se3Cl(Br) Compounds, Proc. Shevchenko Sci. Soc. Chem. Sci., 2022, LXX, p 62-68. https://doi.org/10.37827/ntsh.chem.2022.70.062
V. Kozak, I. Ivashchenko, L. Gulay, and I. Olekseyuk, Crystal Structure of the AgGa2Se3Cl and AgGa2Te3Cl Compounds, Chem. Met. Alloys, 2020, 13, p 45-48.
I.A. Ivashchenko, V.S. Kozak, L.D. Gulay, and I.D. Olekseiuk, The Crystal Structure of the Compound AgGa2Te3I, Uzhhorod Univ. Sci. Bull. Chem. Ser., 2022, 47, p 19-21. https://doi.org/10.24144/2414-0260.2022.1.19-21
Y. Yude, H. Boysen, and H. Schulz, Neutron Powder Investigation of CuI, Z. Kristallogr., 1990, 191, p 79-91. https://doi.org/10.1524/zkri.1990.191.1-2.79
L.D. Gulay, M. Daszkiewicz, O.M. Strok, and A. Pietraszko, Crystal Structure of Cu2Se, Chem. Met. Alloys, 2011, 4, p 200-205. https://doi.org/10.30970/cma4.0184
S. Popovic, A. Tonejc, B. Grzeta-Plencovic, B. Celustka, and R. Trojko, Revised and New Crystal Data for Indium Selenides, J. Appl. Cryst, 1979, 12, p 416. https://doi.org/10.1107/S0021889879012863
O.F. Zmiy, I.A. Mishchenko, and I.D. Olekseyuk, Phase Equilibria in the Quasi-Ternary System Cu2Se-CdSe-In2Se3, J. Alloys Compd., 2004, 367, p 49-57. https://doi.org/10.1016/j.jallcom.2003.08.011
L. Gulay, I. Ivashchenko, O. Zmiy, and I. Olekseyuk, Crystal Structure of the CuIn7Se11 Compound, J. Alloys Compd., 2004, 384, p 121-124. https://doi.org/10.1016/j.jallcom.2004.03.117
I. Ivashchenko, L. Gulay, O. Zmiy, and I. Olekseyuk, The Quasiternary System Cu2Se-CdSe-In2Se3 and Crystal Structure of the Cu0.6Cd0.7In6Se10 Compound, J. Alloys Compd., 2005, 394, p 186-193. https://doi.org/10.1016/j.jallcom.2004.10.031
M.V. Moroz, M.V. Prokhorenko, S.V. Prokhorenko, M.V. Yatskov, and O.V. Reshetnyak, Thermodynamic Properties of AgIn2Te3I and AgIn2Te3Br Determined by EMF Method, J. Phys. Chem., 2018, 92, p 19-23. https://doi.org/10.1134/S0036024418010168
E. Parte, Elements of Inorganic Structural Chemistry, 2nd edn. Geneve, Archive ouverte UNIGE, 1996, p141
H. Hahn, G. Frank, W. Klingler, A.D. Strörger, and G. Strörger, Untersuchungen Über Ternäre Chalkogenide. VI. Über Ternäre Chalcogenide des Aluminiums, Galliums und Indiums mit Zink, Cadmium und Quecksilber, Z. Anorg. Chem, 1955, 279, p 241-270. https://doi.org/10.1002/zaac.19552790502
H. Hahn, G. Frank, and W. Klingler, Zur Struktur des β-Cu2HgJ4 und des β-Ag2HgJ4, Z. Anorg. Chem., 1955, 279, p 271-280. https://doi.org/10.1002/zaac.19552790503
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ivashchenko, I.A., Kozak, V.S., Gulay, L.D. et al. Phase Equilibria in the Quasi-Ternary System Cu2Se-In2Se3-CuI and the Crystal Structure of the AIBIII2XVI3YVII Compounds, Where AI-Cu, Ag; BIII-Ga; XVI-Cl, Br, I; YVII-S, Se, Te. J. Phase Equilib. Diffus. 44, 714–728 (2023). https://doi.org/10.1007/s11669-023-01073-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-023-01073-9