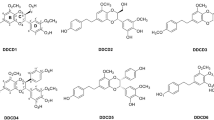
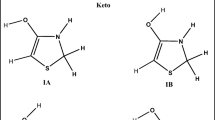
Abstract
The electronic characterization of monomer and dimer molecule’s C6H6O4 was also calculated using the DFT (B3LYP/6311G++(d,p)) method. The calculated TDOS, HOMO, and LUMO were performed through intramolecular charge transfer to investigate the molecule’s stability. In dimethylsulfoxide (DMSO), the UV spectrum has been determined. According to Swiss-ADME, Aspergillus oryzae is not mutagenic or carcinogenic. Additionally calculated were the Mulliken charges, Fukui indices and MEP. Swiss-ADME was used to forecast predictions and significant descriptors of the compounds’ physicochemical characteristics. The intermolecular interactions were examined using topological analysis techniques like RDG and IRI. For the 1JNK Protein, Aspergillus oryzae has a binding affinity of –6.21.




Similar content being viewed by others
REFERENCES
J. D. Bos and M. M. Meinardi, Exp. Dermatol. 9, 165 (2000).
W. J. Balfour, Spectrochim. Acta, Part A 39, 795 (1983).
V. Ashok Babu, B. Lakshmaiah, K. Sree Ramulu, and G. Ramana Rao, Ind. J. Pure Appl. Phys. 25, 58 (1987).
G. A. Burdock, M. G. Soni, and I. G. Carabin, Regulat. Toxicol. Pharmacol. 33, 80 (2001).
H. S. Rho, C. S. Lee, S. M. Ahn, Y. D. Hong, S. S. Shin, Y.-H. Park, et al., Bull. Korean Chem. Soc. 32, 4411 (2011).
M. Naoki, N. Shusuke, T. Satosi, and E. Takeaki, Opt. Commun. 260, 25 (2006).
J. C. Espin, J. Tudela, and F. Garcia-Canovas, Anal. Biochem. 259, 118 (1998).
L. Saghaie, M. Pourfarzam, A. Fassihi, and B. Sartippour, Res. Pharm. Sci. 8, 233 (2013).
M. Mohammadpour, A. Sadeghi, A. Fassihi, L. Saghaei, A. Movahedian, and M. Rostami, Res. Pharm. Sci. 7, 171 (2012).
T. Lu and F. Chen, J. Comput. Chem. 33, 580 (2012).
3D QSAR in Drug Design, Ed. by H. Kubinyi, G. Folkers, and Y. C. Martin (Kluwer, The Netherlands, 1997), Vols. 2, 3.
H. van de Waterbeemd, Structure Property Correlations in Drug Research (Academic, New York, 1996).
The Practice of Medicinal Chemistry, Ed. by C. Wermuth (Academic, New York, 1998).
D. A. Filimonov and V. V. Poroikov, in Bioactie Compound Design: Possibilities for Industrial Use (BIOS Sci., Oxford, 1996), p. 47.
T. A. Gloriozova, D. A. Filimonov, A. A. Lagunin, and V. V. Poroikov, Pharm. Chem. J. 32, 658 (1998).
V. Poroikov and D. Filimonov, in Rational Approaches to Drug Design (Prous Science, Barcelona, 2001), p. 403.
A. Kubaib, P. M. Imran, and A. A. Basha, Comput. Theor. Chem. 1217, 113934 (2022).
R. G. Pearson, Proc. Natl. Acad. Sci. U. S. A. 83, 84408441 (1986).
M. D. Aytemir and G. Karakaya, in Medicinal Chemistry and Drug Design, Ed. by D. Ekinci (InTech Open, Rijeka, 2012).
A. Kubaib and P. M. Imran, J. Mater. Sci. 58, 4005 (2023).
N. O. Eddy, P. O. Ameh, and N. B. Essien, J. Taibah Univ. Sci. 12, 545 (2018).
A. Kubaib and P. M. Imran, J. Indian Chem. Soc. 100, 100804 (2023).
A. A. Basha, F. L. A. Khan, P. Muzammil, and G. S. Fasiuddin, Mater. Res. Express 9, 075303 (2022).
M. Yeo, P. M. Niamien, E. B. A. Bilé, and A. Trokourey, J. Comput. Methods Mol. Des. 8, 13 (2018).
M. Yeo, P. M. Niamien, E. B. A. Bilé, and A. Trokourey, J. Comput. Methods Mol. Des. 8, 13 (2018).
Y. S. D. N’Guessan, G. G. D. Diomandé, S. J. Akpa, A. Ouédraogo, L. A. G. Pohan, P. M. Niamien, and A. Trokourey, Int. J. Appl. Pharm. Sci. Res. 3 (4), 41 (2018).
A. A. Basha et al., J. Mol. Liq. 363, 119853 (2022).
Y. S. D. N’Guessan, G. G. D. Diomandé, S. J. Akpa, A. Ouédraogo, L. A. G. Pohan, P. M. Niamien, and A. Trokourey, Int. J. Appl. Pharm. Sci. Res. 3 (4), 41 (2018).
N. O. Eddy, P. O. Ameh, and N. B. Essien, J. Taibah Univ. Sci. 12, 545 (2018).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Attar Kubaib: conceptualization, methodology, validation, data curation, writing–original draft. Predhanekar Mohamed Imran: supervisor, formal analysis, software. K. Anandaratchagan: investigation, visualization, resource. M. Selvakumaran: writing–review and editing. S. Sulthanudeen: investigation. S. Sanjeev: visualization. A. Aathif Basha: resource.
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Kubaib, A., Imran, P.M., Anandaratchagan, K. et al. Chelation Agent As Potential Target Antioxidant: DFT, Physicochemical Properties, Topological Analysis, and Molecular Docking Studies into Intramolecular Interactions. Russ. J. Phys. Chem. 97, 2884–2893 (2023). https://doi.org/10.1134/S0036024423120038
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423120038