Abstract
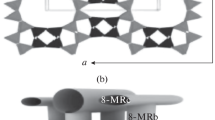
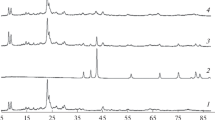
The adsorption, temperature-programmed desorption, and carbonylation of dimethyl ether (DME) in the presence of mordenite and ferrierite (SiO2/Al2O3 ≈ 20, Zeolyst International) is studied. The effect of introducing Cu, Co, and Mg cations by ion exchange is discussed. Dimethyl ether carbonylation is conducted at 200°С, a pressure of 3 MPa, and a space velocity of 8000 mL g–1 h–1 in the following mixture (vol %): DME, ⁓2.2; CO, 92.8–95.5; and the rest, N2. After an induction period, the methyl acetate content in the presence of mordenite is about 4–5 times higher than that in the presence of ferrierite. Water, methanol, and hydrocarbons are formed in trace amounts. The introduction of Cu, Co, and Mg cations into mordenite by ion exchange (single ion-exchange run, cation/Al ratio of no more than 35%) leads to an increase not only in stability, but also in activity in the DME carbonylation reaction. It is found that an increase in the content of copper (from 1.19 to 2.23 wt %) and magnesium (from 0.62 to 1.8 wt %) has different effects on activity. It increases in the case of copper and decreases in the case of magnesium. The prereduction of a copper-exchanged mordenite leads to the appearance of metallic copper particles on the surface of the mordenite crystallites and a decrease in activity. According to in situ diffuse reflectance infrared spectroscopy, the introduction of magnesium cations by three ion-exchange runs leads to a significant decrease in the number of Brønsted acid sites (BASes) in both the 12-MR and 8-MR channels of the mordenite. The catalytic characteristics of ferrierite hardly change upon the introduction of copper and magnesium by ion exchange.






Similar content being viewed by others
REFERENCES
Cheung, P., Bhan, A., Sunley, G.J., and Iglesia, E., Angew. Chem., 2006, vol. 118, p. 1647.
Bhan, A., Allian, A.D., Sunley, G.J., Law, D.J., and Iglesia, E., J. Am. Chem. Soc., 2007, vol. 129, p. 4919.
Volnina, E.A., Kipnis, M.A., and Khadzhiev, S.N., Petrol. Chem., 2017, vol. 57, no. 5, p. 353.
Kipnis, M.A. and Volnina, E.A., Kinet. Catal., 2022, vol. 63, p. 129.
Zhan, E., Xiong, Z., and Shen, W., J. Energy Chem., 2019, vol. 36, p. 51.
Le, T.T., Chawla, A., and Rimer, J.D., J. Catal., 2020, vol. 391, p. 56.
Alberti, A., Zeolites, 1997, vol. 19, p. 411.
Simoncic, P. and Armbruster, T., Am. Mineral., 2004, vol. 89, p. 421.
Kerr, I.S., Nature, 1966, vol. 210, p. 294.
Guo, W., Zhu, L., Wang, H., Qiu, K., and Cen, K., J. Phys. Chem. C, 2015, vol. 119, p. 524.
Li, Y., Huang, S., Cheng, Z., Wang, S., Ge, Q., Ma, X, J. Catal., 2018, vol. 365, p. 440.
Cheng, Z., Huang, S., Li, Y., Cai, K., Yao, D., Lv, J., Wang, S., and Ma, X., Appl. Catal. A: Gen., 2019, vol. 576, p. 1.
Zhan, H., Huang, S., Li, Y., Lv, J., Wang, S., and Ma, X., Catal. Sci. Technol., 2015, vol. 5, p. 4378.
Reule, A.A.C., Semagina, N., ACS Catal., 2016, vol. 6, p. 4972.
Reule, A.A.C., Prasad, V., and Semagina, N., Micropor. Mesopor. Mater., 2018, vol. 263, p. 220.
Blasco, T., Boronat, M., Concepcion, P., Corma, A., Law, D., and Vidal-Moya, J.A., Angew. Chem., Int. Ed. Engl., 2007, vol. 46, p. 3938.
Ma, M., Zhan, E., Huang, X., Ta, N., Xiong, Z., and Bai, L., Shen, W., Catal. Sci. Technol., 2018, vol. 8, p. 2124.
Xu, F., Hong, Z., Lv, J., Chen, C., Zhao, G., Miao, L., Yang, W., and Zhu, Z., Appl. Catal. A: Gen., 2022, vol. 648, p. 118928.
Kipnis, M.A., Samokhin, P.V., Yashina, O.V., and Sukhorebrova, O.A., Russ. J. Phys. Chem. A, 2013, vol. 87, no. 5, p. 851.
Kipnis, M.A., Belostotskii, I.A., Volnina, E.A., Lin, G.I., Marshev, I.I, Kinet. Catal., 2018, vol. 59, p. 754.
Samokhin, P.V., Belostotskii, I.A., Marshev, I.I., and Kipnis M.A., J. Anal. Chem., 2019, vol. 74, no. Suppl. 2, p. 17.
Rasmussen, D.B., Christensen, J.M., Temel, B., Studt, F., Moses, P.G., Rossmeisl, J., Riisager, A., and Jensen, A.D., Catal. Sci. Technol., 2017, vol. 7, p. 1141.
Feng, P., Zhang, G., Chen, X., Zang, K., and Li, X., Xu, L., Appl. Catal. A: Gen., 2018, vol. 557, p. 119.
Kim, E.J., Gao, X., Tian, J., and Bae, J.W., Catal. Today, 2023, vols. 411–412, p. 113822.
Cherkasov, N., Vazhnova, T., and Lukyanov, D.B., Vib. Spectrosc., 2016, vol. 83, p. 170.
Little, L.H., Infrared Spectra of Adsorbed Species, London: Academic Press, 1966.
Theivasanthi, T. and Alagar, M., Arch. Phys. Res., 2010, vol. 1, no. 2, p. 112.
Reule, A.A.C., Shen, J., and Semagina, N., ChemPhysChem, 2018, vol. 19, p. 1500.
ACKNOWLEDGMENTS
This work was performed at Topchiev Institute of Petrochemical Synthesis of the Russian Academy of Sciences using the equipment of the Center for collective use of this institute.
The authors thank A.R. Kubareva for participation in adsorption measurements and V.S. Ryleev for determining the morphology of the samples by TEM.
Funding
This work was performed under a state task to Topchiev Institute of Petrochemical Synthesis of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by M. Timoshinina
Abbreviations and notation: BAS, Brønsted acid site; BET, Brunauer–Emmett–Teller method; DME, dimethyl ether; MA, methyl acetate; H-FER, ferrierite in the proton form; H-MOR, mordenite in the proton form; n-MR, n-membered ring; GHSV, gas hourly space velocity; TEM, transmission electron microscopy; IR, infrared; DRIFTS, diffuse reflectance infrared Fourier transform spectroscopy; XRD, X-ray diffraction analysis; DFT, density functional theory; EELS, electron energy loss spectroscopy; EFTEM, energy-filtered transmission electron microscopy; EDS, energy-dispersive X-ray spectroscopy.
Rights and permissions
About this article
Cite this article
Kipnis, M.A., Galkin, R.S., Volnina, E.A. et al. Dimethyl Ether Carbonylation in the Presence of an H-MOR Zeolite Modified with Copper, Cobalt, and Magnesium. Kinet Catal 64, 849–861 (2023). https://doi.org/10.1134/S0023158423060071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158423060071