Abstract
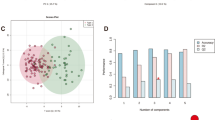
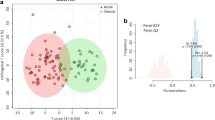
Brain stroke (BS, also known as a cerebrovascular accident), represents a serious global health crisis. It has been a leading cause of permanent disability and unfortunately, frequent fatalities due to lack of timely medical intervention. While progress has been made in prevention and management, the complexities and consequences of stroke continue to pose significant challenges, especially, its impact on patient’s quality of life and independence. During stroke, there is a substantial decrease in oxygen supply to the brain leading to alteration of cellular metabolic pathways, including those involved in mitochondrial-damage, leading to mitochondrial-dysfunction. The present proof-of-the-concept metabolomics study has been performed to gain insights into the metabolic pathways altered following a brain stroke and discover new potential targets for timely interventions to mitigate the effects of cellular and mitochondrial damage in BS. The serum metabolic profiles of 108 BS-patients were measured using 800 MHz NMR spectroscopy and compared with 60 age and sex matched normal control (NC) subjects. Compared to NC, the serum levels of glutamate, TCA-cycle intermediates (such as citrate, succinate, etc.), and membrane metabolites (betaine, choline, etc.) were found to be decreased BS patients, whereas those of methionine, mannose, mannitol, phenylalanine, urea, creatine and organic acids (such as 3-hydroxybutyrate and acetone) were found to be elevated in BS patients. These metabolic changes hinted towards hypoxia mediated mitochondrial dysfunction in BS-patients. Further, the area under receiver operating characteristic curve (ROC) values for five metabolic features (methionine, mannitol, phenylalanine, mannose and urea) found to be more than 0.9 suggesting their high sensitivity and specificity for differentiating BS from NC subjects.
Graphical abstract





Similar content being viewed by others
Data availability
The manuscript contains all the relevant data with its supporting information files. The corresponding authors will be provided with raw NMR spectral data upon request. The raw NMR data has been uploaded on public repository ZENODO (Accession Number: 7792273 | https://doi.org/10.5281/zenodo.7046041).
Abbreviations
- NMR :
-
Nuclear Magnetic Resonance
- BS :
-
Brain Stroke
- IS :
-
Ischemic stroke
- MRI :
-
Magnetic Resonance Imaging
- CVD :
-
Cardiovascular disease
- CPMG :
-
Carr–Purcell–Meiboom–Gill
- BCAA :
-
Branched‐chain amino acid
- BTR :
-
Branched chain amino acids-to-tyrosine ratio
- PTR :
-
Phenylalanine-to-Tyrosine ratio
- HTR :
-
Histidine-to-Tyrosine ratio
- CI :
-
Confidence interval
- ICU :
-
Intensive care unit
- 3-HB :
-
3-Hydroxy-isobutyrate
- TSP :
-
Trimethylsilylpropionic acid
- NAG :
-
N-acetyl glycoprotein
- FDA :
-
Food and Drug Administration
- MHz :
-
Megahertz
- NC :
-
Normal control
- HS :
-
Hemorrhagic stroke
- ICH :
-
Intracerebral hemorrhage
- PLS-DA :
-
Partial least square-discriminant analysis
- PCA :
-
Principal component analysis
- MDA :
-
Mean decrease accuracy
- RF :
-
Random forest
- ROC :
-
Receiver operating characteristic
- AUROC :
-
Area under ROC curve
- ESM :
-
Electronic Supplementary Material
- 1D/2D :
-
One/Two dimensional
- CT :
-
Computerized tomography
- LDL :
-
Low-density lipoproteins
- VLDL :
-
Very Low-density lipoproteins
- HDL :
-
High density lipoprotein
References
Allen CL, Bayraktutan U (2009) Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 4:461–470
Arya P, Kumar U, Sharma S, Durgappa M, Guleria A, Raj R, Pande G, Kumar D (2021) Targeted NMR-based serum metabolic profiling of serine, glycine and methionine in acute-on-chronic liver failure patients: possible insights into mitochondrial dysfunction. Anal Sci Adv 2:536–545
Au A (2018) Metabolomics and lipidomics of ischemic stroke. Adv Clin Chem 85:31–69
Baek SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim ES, Yu SW, Majid A, Bae ON (2014) Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke 45:2438–2443
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, De Ferranti SD, Floyd J, Fornage M, Gillespie C (2017) Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135:e146–e603
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R (2018) Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137:e67–e492
Biran V, Cochois V, Karroubi A, Arrang JM, Charriaut-Marlangue C, Heron A (2008) Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol 18(1):1–9
Carinci M, Vezzani B, Patergnani S, Ludewig P, Lessmann K, Magnus T, Casetta I, Pugliatti M, Pinton P, Giorgi C (2021) Different roles of mitochondria in cell death and inflammation: focusing on mitochondrial quality control in ischemic stroke and reperfusion. Biomedicines 9:169
Chan PH (2005) Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann N Y Acad Sci 1042:203–209
Chei CL, Yamagishi K, Kitamura A, Kiyama M, Imano H, Ohira T, Cui R, Tanigawa T, Sankai T, Ishikawa Y (2013) High-density lipoprotein subclasses and risk of stroke and its subtypes in Japanese population: the Circulatory Risk in Communities Study. Stroke 44:327–333
Chen HJ, Shen YC, Lin CY, Tsai KC, Lu CK, Shen CC, Lin YL (2012) Metabolomics study of Buyang Huanwu Tang Decoction in ischemic stroke mice by 1 H NMR. Metabolomics 8:974–984
Chen S, Dong Z, Zhao Y, Sai N, Wang X, Liu H, Huang G, Zhang X (2017) Homocysteine induces mitochondrial dysfunction involving the crosstalk between oxidative stress and mitochondrial pSTAT3 in rat ischemic brain. Sci Rep 7:6932
Demine S, Reddy N, Renard P, Raes M, Arnould T (2014) Unraveling biochemical pathways affected by mitochondrial dysfunctions using metabolomic approaches. Metabolites 4:831–878
Durand P, Prost M, Loreau N, Lussier-Cacan S, Blache D (2001) Impaired homocysteine metabolism and atherothrombotic disease. Lab Invest 81:645–672
Fowler B (2005) Homocysteine: overview of biochemistry, molecular biology, and role in disease processes. Semin Vasc Med 5(02):77–86 (Copyright-¬ 2005 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New-áª. Ref Type: Conference Proceeding)
Gao J, Yang H, Chen J, Fang J, Chen C, Liang R, Yang G, Wu H, Wu C, Li S (2013) Analysis of serum metabolites for the discovery of amino acid biomarkers and the effect of Galangin on cerebral ischemia. Mol BioSyst 9:2311–2321
Geisler S, Gostner JM, Becker K, Ueberall F, Fuchs D (2013) Immune activation and inflammation increase the plasma phenylalanine-to-tyrosine ratio. Pteridines 24:27–31
Gibney MJ, Walsh M, Brennan L, Roche HM, German B, Van Ommen B (2005) Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr 82:497–503
Glushakova OY, Glushakov AV, Miller ER, Valadka AB, Hayes RL (2016) Biomarkers for acute diagnosis and management of stroke in neurointensive care units. Brain Circ 2:28
Gowda GN, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D (2008) Metabolomics-based methods for early disease diagnostics. Expert Rev Mol Diagn 8:617–633
Grandizoli CW, Lange MC, Novak F, Campos FR, Barison A (2014) Ischemic stroke progress evaluation by 31P NMR-based metabonomic of human serum. J Braz Chem Soc 25:1143–1149
Guleria A, Kumar A, Kumar U, Raj R, Kumar D (2018) NMR based metabolomics: an exquisite and facile method for evaluating therapeutic efficacy and screening drug toxicity. Curr Top Med Chem 18:1827–1849
Gupta S, Sharma U (2021) Metabolomics of neurological disorders in India. Anal Sci Adv 2:594–610
Gupta N, Yadav DK, Gautam S, Kumar A, Kumar D, Prasad N (2023) Nuclear magnetic resonance-based metabolomics approach revealed the intervention effect of using Complementary and Alternative Medicine (CAM) by CKD patients. ACS Omega 8:7722–7737
Hirasawa N (2019) Expression of histidine decarboxylase and its roles in inflammation. Int J Mol Sci 20:376
Holecek M (2020) Histidine in health and disease: metabolism, physiological importance, and use as a supplement. Nutrients 12:848
Howard VJ, Sides EG, Newman GC, Cohen SN, Howard G, Malinow MR, Toole JF (2002) Changes in plasma homocyst (e) ine in the acute phase after stroke. Stroke 33:473–478
Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, Imano H, Okamura T, Naito Y, Shimamoto T (2002) Linoleic acid, other fatty acids, and the risk of stroke. Stroke 33:2086–2093
Jayachitra S, Prasanth A (2021) Multi-feature analysis for automated brain stroke classification using weighted Gaussian naive Bayes classifier. J Circ Syst Comput 30:2150178
Jia J, Zhang H, Liang X et al (2021) Application of Metabolomics to the Discovery of Biomarkers for Ischemic Stroke in the Murine Model: a Comparison with the Clinical Results. Mol Neurobiol 58:6415–6426. https://doi.org/10.1007/s12035-021-02535-2
Jung JY, Lee HS, Kang DG, Kim NS, Cha MH, Bang OS, Ryu DH, Hwang GS (2011) 1H-NMR-based metabolomics study of cerebral infarction. Stroke 42:1282–1288
Koek MM, Jellema RH, van der Greef J, Tas AC, Hankemeier T (2011) Quantitative metabolomics based on gas chromatography mass spectrometry: status and perspectives. Metabolomics 7:307–328
Kumar U, Jain A, Guleria A, Misra DP, Goel R, Danda D, Misra R, Kumar D (2020) Circulatory glutamine/glucose ratio for evaluating disease activity in Takayasu arteritis: a NMR based serum metabolomics study. J Pharm Biomed Anal 180:113080
Kusum K, Raj R, Rai S, Pranjali P, Ashish A, Vicente-Muñoz S, Chaube R, Kumar D (2022) Elevated Circulatory Proline to Glutamine Ratio (PQR) in endometriosis and its potential as a diagnostic biomarker. ACS Omega 7(17):14856–14866
Lee Y, Khan A, Hong S, Jee SH, Park YH (2017) A metabolomic study on high-risk stroke patients determines low levels of serum lysine metabolites: a retrospective cohort study. Mol BioSyst 13:1109–1120
Li N, Kong X, Ye R, Yang Q, Han J, Xiong L (2011) Age-related differences in experimental stroke: possible involvement of mitochondrial dysfunction and oxidative damage. Rejuvenation Res 14:261–273
Li Q, Zhang T, Wang J, Zhang Z, Zhai Y, Yang GY, Sun X (2014) Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochem Biophys Res Commun 444:182–188
Li W, Shao C, Zhou H, Du H, Chen H, Wan H, He Y (2022) Multi-omics research strategies in ischemic stroke: A multidimensional perspective. Ageing Res Rev 81:101730
Li Q, Gao S (2017) Mitochondrial dysfunction in ischemic stroke. In: Lapchak P, Yang GY (eds) Translational research in stroke. Translational Medicine Research. Springer, Singapore. https://doi.org/10.1007/978-981-10-5804-2_10
Liu M, Zhou K, Li H, Dong X, Tan G, Chai Y, Wang W, Bi X (2015) Potential of serum metabolites for diagnosing post-stroke cognitive impairment. Mol BioSyst 11:3287–3296
Liu P, Li R, Antonov AA, Wang L, Li W, Hua Y, Guo H, Wang L, Liu P, Chen L (2017) Discovery of metabolite biomarkers for acute ischemic stroke progression. J Proteome Res 16:773–779
Liu F, Lu J, Manaenko A, Tang J, Hu Q (2018) Mitochondria in ischemic stroke: new insight and implications. Aging Dis 9:924
LRoS Collaborators GBD, Feigin VL, Nguyen G et al (2018) Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Eng J Med 379:2429–2437
Luo L, Kang J, He Q, Qi Y, Chen X, Wang S, Liang S (2019) A NMR-based metabonomics approach to determine protective effect of a combination of multiple components derived from Naodesheng on ischemic stroke rats. Molecules 24(9):1831
Miller AL (2003) The methionine-homocysteine cycle and its effects on cognitive diseases. (Homocysteine & Cognitive). Altern Med Rev 8(1):7–20
Mizuno T, Hoshino T, Ishizuka K, Toi S, Takahashi S, Wako S, Arai S, Kitagawa K (2023) Hyperhomocysteinemia Increases Vascular Risk in Stroke Patients with Chronic Kidney Disease. J Atheroscler Thromb 30(9):1198–1209. https://www.jstage.jst.go.jp/article/jat/30/9/30_63849/_article/-char/en
Moreau R, Clária J, Aguilar F, Fenaille F, Lozano JJ, Junot C, Colsch B, Caraceni P, Trebicka J, Pavesi M (2020) Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol 72:688–701
Muhammed H, Kumar D, Dubey D, Kumar S, Chaurasia S, Guleria A, Majumder S, Singh R, Agarwal V, Misra R (2020) Metabolomics analysis revealed significantly higher synovial Phe/Tyr ratio in reactive arthritis and undifferentiated spondyloarthropathy. Rheumatology 59:1587–1590
Murr C, Grammer TB, Meinitzer A, Kleber ME, März W, Fuchs D (2014) Immune activation and inflammation in patients with cardiovascular disease are associated with higher phenylalanine to tyrosine ratios: the ludwigshafen risk and cardiovascular health study. J Amino Acids 2014:1–6
Musuka TD, Wilton SB, Traboulsi M, Hill MD (2015) Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ 187:887–893
Niizuma K, Endo H, Chan PH (2009) Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem 109:133–138
Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, Okami N, Chan PH (2010) Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochimica et Biophysica Acta (BBA)-Mol Basis Dis 1802:92–99
Norat P, Soldozy S, Sokolowski JD, Gorick CM, Kumar JS, Chae Y, Yağmurlu K, Prada F, Walker M, Levitt MR (2020) Mitochondrial dysfunction in neurological disorders: Exploring mitochondrial transplantation. NPJ Regen Med 5:22
Norrving B, Mensah GA (2017) Global burden of stroke. Circ Res 120:439–448
Ormstad H, Verkerk R, Sandvik L (2016) Serum phenylalanine, tyrosine, and their ratio in acute ischemic stroke: on the trail of a biomarker? J Mol Neurosci 58:102–108
Piantadosi CA, Zhang J (1996) Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke 27:327–332
Rout M, Vaughan A, Blair A, Stavrakis S, Sidorov EV, Sanghera DK (2023) Discovery and validation of circulating stroke metabolites by NMR-based analyses using patients from the MISS and UK Biobank. Neurochem Int 169:105588
Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G (2006) Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J 20:1064–1073
Sarmah D, Kaur H, Saraf J, Vats K, Pravalika K, Wanve M, Kalia K, Borah A, Kumar A, Wang X (2019) Mitochondrial dysfunction in stroke: implications of stem cell therapy. Transl Stroke Res 10:121–136
Schrock JW, Glasenapp M, Drogell K (2012) Elevated blood urea nitrogen/creatinine ratio is associated with poor outcome in patients with ischemic stroke. Clin Neurol Neurosurg 114:881–884
Shin TH, Lee DY, Basith S, Manavalan B, Paik MJ, Rybinnik I, Mouradian MM, Ahn JH, Lee G (2020) Metabolome changes in cerebral ischemia. Cells 9:1630
Sidorov E, Sanghera DK, Vanamala JK (2019) Biomarker for ischemic stroke using metabolome: a clinician perspective. J Stroke 21:31
Sidorov EV, Rout M, Xu C, Jordan L, Fields E, Apple B, Kyle S, Gordon D, Chainakul J, Sanghera DK (2023) Difference in acute and chronic stage ischemic stroke metabolic markers with controls. J Stroke Cerebrovasc Dis 32:107211
Singh A, Prakash V, Gupta N, Kumar A, Kant R, Kumar D (2022) Serum metabolic disturbances in lung cancer investigated through an elaborative NMR-based serum metabolomics approach. ACS Omega 7:5510–5520
Tao S, Xiao X, Li X, Na F, Na G, Wang S, Zhang P, Hao F, Zhao P, Guo D (2023) Targeted metabolomics reveals serum changes of amino acids in mild to moderate ischemic stroke and stroke mimics. Front Neurol 14:1153193
Tian H, Chen X, Liao J, Yang T, Cheng S, Mei Z, Ge J (2022) Mitochondrial quality control in stroke: from the mechanisms to therapeutic potentials. J Cell Mol Med 26:1000–1012
Vinknes KJ, Refsum H, Turner C, Khaw KT, Wareham NJ, Forouhi NG, Imamura F (2021) Plasma sulfur amino acids and risk of cerebrovascular diseases: A nested case-control study in the EPIC-Norfolk cohort. Stroke 52:172–180
Virtanen JK, Voutilainen S, Rissanen TH, Happonen P, Mursu J, Laukkanen JA, Poulsen H, Lakka TA, Salonen JT (2006) High dietary methionine intake increases the risk of acute coronary events in middle-aged men. Nutr Metab Cardiovasc Dis 16:113–120
Vojinovic D, Kalaoja M, Trompet S, Fischer K, Shipley MJ, Li S, Havulinna AS, Perola M, Salomaa V, Yang Q (2021) Association of circulating metabolites in plasma or serum and risk of stroke: meta-analysis from 7 prospective cohorts. Neurology 96:e1110–e1123
Vosler PS, Graham SH, Wechsler LR, Chen J (2009) Mitochondrial targets for stroke: focusing basic science research toward development of clinically translatable therapeutics. Stroke 40:3149–3155
Wang ZY, Sun ZR, Zhang LM (2014) The relationship between serum mannose-binding lectin levels and acute ischemic stroke risk. Neurochem Res 39:248–253
Wang D, Kong J, Wu J, Wang X, Lai M (2017) GC-MS-based metabolomics identifies an amino acid signature of acute ischemic stroke. Neurosci Lett 642:7–13
Wang M, Gui X, Wu L, Tian S, Wang H, Xie L, Wu W (2020) Amino acid metabolism, lipid metabolism, and oxidative stress are associated with post-stroke depression: a metabonomics study. BMC Neurol 20:1–10
Wang X, Zhang L, Sun W, Pei Ll, Tian M, Liang J, Liu X, Zhang R, Fang H, Wu J (2021) Changes of metabolites in acute ischemic stroke and its subtypes. Front Neurosci 14:580929
Wang B-L, Wu J-F, Xiao D, Wu B, Wei D-X (2023) 3-hydroxybutyrate in the brain: biosynthesis, function, and disease therapy. Brain-X 1:e6
Wen L, Yan C, Zheng W, Li Y, Wang Y, Qu M (2023) Metabolic alterations and related biological functions of post-stroke depression in ischemic stroke patients. Neuropsychiatr Dis Treat 19:1555–1564
Zhang R, Meng J, Wang X, Pu L, Zhao T, Huang Y, Han L (2022) Metabolomics of ischemic stroke: insights into risk prediction and mechanisms. Metab Brain Dis 37:2163–2180
Zhao T, Yan Q, Wang C, Zeng J, Zhang R, Wang H, Pu L, Dai X, Liu H, Han L (2023) Identification of serum biomarkers of ischemic stroke in a hypertensive population based on metabolomics and lipidomics. Neuroscience 533:22–35
Acknowledgements
Authors would like to acknowledge the Department of Medical Education, Govt. of Uttar Pradesh for supporting the High Field NMR Facility at Centre of Biomedical Research, Lucknow, India. AK acknowledges the financial assistance from Department of Science and Technology, Govt of India support scheme DST/CSRI/PDF-63/2018 under Cognitive Science Research Initiative program. GS acknowledges receipt of the JRF fellowship from The CSIR, New Delhi, India. The manuscript communication number received from Integral University is IU/R&D/2023-MCN0002286.
Funding
(1). Intramural funding from the Centre of Biomedical Research (CBMR), Lucknow (Project No. CBMR/ IMR/0010/2021 | PI: Dr. Dinesh Kumar). (2). Department of Science and Technology, Govt of India support scheme DST/CSRI/PDF-63/2018 under Cognitive Science Research Initiative program. (3). Department of Science and Technology for financial assistance under SERB EMR Scheme (Ref. No.: EMR/2016/001756). (4). Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Author information
Authors and Affiliations
Contributions
DK and AK: Conceptualization of idea and study design.
RNC: Involved in the clinical screening of patients, imaging analysis and clinical data collection.
AK, SS and SA: Collection of blood samples, extraction of serum and compiling the clinical and demographic details.
SY and GS: Prepared NMR buffer and NMR samples, recorded the NMR spectra and performed the concentration profiling.
ARK: Manuscript proof reading, review, and submission.
DK and SY: Analyzed the metabolomics data, prepared the figures, and drafted the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Institute of Medical Sciences, Banaras Hindu University, Varanasi, India with ethical consent no- Dean/2021/EC/2746.
Consent for publication
All the authors agree for publication in the journal.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, S., Kumar, A., Singh, S. et al. NMR based Serum metabolomics revealed metabolic signatures associated with oxidative stress and mitochondrial damage in brain stroke. Metab Brain Dis 39, 283–294 (2024). https://doi.org/10.1007/s11011-023-01331-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-023-01331-2