Abstract
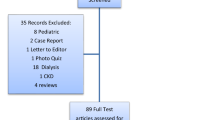
Acute kidney injury (AKI) is a commonly reported adverse effect of administration of antimicrobials. While AKI can be associated with poorer outcomes, there is little information available to understand rates of AKI in children exposed to various antimicrobials. We performed a structured review using the PubMed and Embase databases. Articles were included if they provided an AKI definition in patients who were < 19 years of age receiving an antimicrobial and reported the frequency of AKI. Author-defined AKI rates were calculated for each study and mean pooled estimates for each antimicrobial were derived from among all study participants. Pooled estimates were also derived for those studies that reported AKI according to pRIFLE (pediatric risk, injury, failure, loss, end stage criteria), AKIN (acute kidney injury network), or KDIGO (kidney disease improving global outcomes) creatinine criteria. A total of 122 studies evaluating 28 antimicrobials met the inclusion criteria. Vancomycin was the most commonly studied drug: 11,514 courses across 44 included studies. Among the 27,285 antimicrobial exposures, the overall AKI rate was 13.2% (range 0–42.1% by drug), but the rate of AKI varied widely across studies (range 0–68.8%). Cidofovir (42.1%) and conventional amphotericin B (37.0%) had the highest pooled rates of author-defined AKI. Eighty-one studies used pRIFLE, AKIN, or KDIGO AKI criteria and the pooled rates of AKI were similar to author-defined AKI rates. In conclusion, antimicrobial-associated AKI is reported to occur frequently in children, but the rates of AKI varies widely across studies and drugs. Most published studies examined hospitalized patients and heterogeneity in study populations and in author definitions of AKI are barriers to a comparison of nephrotoxicity risk among antimicrobials in children.



Similar content being viewed by others
Data availability
Data is not available in a repository but authors can provide upon request.
References
Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52. https://doi.org/10.1038/s41572-021-00284-z.
Perazella MA, Rosner MH. Drug-induced acute kidney injury. Clin J Am Soc Neprol. 2022;17(8):1220–33. https://doi.org/10.2215/CJN.11290821.
Downes KJ, Hayes M, Fitzgerald JC, et al. Mechanisms of antimicrobial-induced nephrotoxicity in children. J Antimicrob Chemother. 2020;75(1):1–13. https://doi.org/10.1093/jac/dkz325.
Gerber JS, Newland JG, Coffin SE, et al. Variability in antibiotic use at children’s hospitals. Pediatrics. 2010;126(6):1067–73. https://doi.org/10.1542/peds.2010-1275.
Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130(1):23–31. https://doi.org/10.1542/peds.2011-2879.
Pierson-Marchandise M, Gras V, Moragny J, et al. The drugs that mostly frequently induce acute kidney injury: a case-noncase study of a pharmacovigilance database. Br J Clin Pharmacol. 2017;83(6):1341–9. https://doi.org/10.1111/bcp.13216.
Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–61. https://doi.org/10.2215/CJN.01900214.
Parikh RV, Tan TC, Salyer AS, et al. Community-based epidemiology of hospitalized acute kidney injury. Pediatrics. 2020;146(3): e20192821. https://doi.org/10.1542/peds.2019-2821.
Kaddourah A, Basu RK, Bagshaw SM, Goldstein S, AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11–20. https://doi.org/10.1056/NEJMoa1611391.
Uber AM, Sutherland SM. Acute kidney injury in hospitalized children: consequences and outcomes. Pediatr Nephrol. 2020;35(2):213–20. https://doi.org/10.1007/s00467-018-4128-7.
Lewis SJ, Mueller BA. Antibiotic dosing in patients with acute kidney injury: “enough but not too much.” J Intensive Care Med. 2016;31(3):164–76. https://doi.org/10.1177/0885066614555490.
Kim JY, Yee J, Yoon HY, Han JM, Gwak HS. Risk factors for vancomycin-associated acute kidney injury: a systematic review and meta-analysis. Br J Clin Pharmacol. 2022;88(9):3977–89. https://doi.org/10.1111/bcp.15429.
Kwiatkowska E, Domański L, Dziedziejko V, Kajdy A, Stefańska K, Kwiatkowski S. The mechanism of drug nephrotoxicity and the methods for preventing kidney damage. Int J Mol Sci. 2021;22(11):6109. https://doi.org/10.3390/ijms22116109.
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–35. https://doi.org/10.1038/sj.ki.5002231.
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. https://doi.org/10.1186/cc5713.
Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. https://doi.org/10.1038/kisup.2012.1.
Goswami E, Ogden RK, Bennett WE, et al. Evidence-based development of a nephrotoxic medication list to screen for acute kidney injury risk in hospitalized children. Am J Health Syst Pharm. 2019;76(22):1869–74. https://doi.org/10.1093/ajhp/zxz203.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Downes KJ, Goldman JL. Too much of a good thing: defining antimicrobial therapeutic targets to minimize toxicity. Clin Pharmacol Ther. 2021;109(4):905–17. https://doi.org/10.1002/cpt.2190.
Downes KJ, Cowden C, Laskin BL, et al. Association of acute kidney injury with concomitant vancomycin and piperacillin/tazobactam treatment among hospitalized children. JAMA Pediatr. 2017;171(12): e173219. https://doi.org/10.1001/jamapediatrics.2017.3219.
Hundeshagen G, Herndon DN, Capek KD, et al. Co-administration of vancomycin and piperacillin-tazobactam is associated with increased renal dysfunction in adult and pediatric burn patients. Crit Care. 2017;21(1):318. https://doi.org/10.1186/s13054-017-1899-3.
Kalligeros M, Karageorgos SA, Shehadeh F, Zacharioudakis IM, Mylonakis E. The association of acute kidney injury with the concomitant use of vancomycin and piperacillin/tazobactam in children: a systematic review and meta-analysis. Antimicrob Agents Chemother. 2019;63(12):e01572-19, AAC.01572-19. https://doi.org/10.1128/AAC.01572-19.
Miano TA, Hennessy S, Yang W, et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48(9):1144–55. https://doi.org/10.1007/s00134-022-06811-0.
Pais GM, Liu J, Avedissian SN, et al. Lack of synergistic nephrotoxicity between vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother. 2020;75(5):1228–36. https://doi.org/10.1093/jac/dkz563.
Lu H, Thurnherr E, Meaney CJ, Fusco NM. Incidence and risk factors for acute kidney injury in hospitalized children receiving piperacillintTazobactam. J Pediatr Pharmacol Ther. 2021;26(6):597–602. https://doi.org/10.5863/1551-6776-26.6.597.
Ericson JE, Gostelow M, Autmizguine J, et al. Safety of high-dose acyclovir in infants with suspected and confirmed neonatal herpes simplex virus infections. Pediatr Infect Dis J. 2017;36(4):369–73. https://doi.org/10.1097/INF.0000000000001451.
Rhone ET, Carmody JB, Swanson JR, Charlton JR. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med. 2014;27(14):1485–90. https://doi.org/10.3109/14767058.2013.860522.
Rao S, Abzug MJ, Carosone-Link P, et al. Intravenous acyclovir and renal dysfunction in children: a matched case control study. J Pediatr. 2015;166(6):1462-8.e4. https://doi.org/10.1016/j.jpeds.2015.01.023.
Kendrick JG, Ensom MHH, Steer A, White CT, Kwan E, Carr RR. Standard-dose versus high-dose acyclovir in children treated empirically for encephalitis: a retrospective cohort study of its use and safety. Pediatr Drugs. 2014;16(3):229–34. https://doi.org/10.1007/s40272-014-0066-4.
Downes KJ, Boge CLK, Baro E, et al. Acute kidney injury during treatment with intravenous acyclovir for suspected or confirmed neonatal herpes simplex virus infection. J Pediatr. 2020;219:126-32.e2. https://doi.org/10.1016/j.jpeds.2019.12.056.
Sandery BJ, Erlich JH, Kennedy SE. Acute kidney injury following intravenous acyclovir in children. Arch Dis Child. 2020;105(12):1215–9. https://doi.org/10.1136/archdischild-2019-317990.
Yalçınkaya R, Öz FN, Kaman A, et al. Factors associated with acyclovir nephrotoxicity in children: data from 472 pediatric patients from the last 10 years. Eur J Pediatr. 2021;180(8):2521–7. https://doi.org/10.1007/s00431-021-04093-0.
Sonia SF, Hassan MS, Ara F, Hanif M. Fractional excretion of magnesium, a marker of aminoglycoside induced nephrotoxicity in neonates. Saudi J Kidney Dis Transpl. 2016;27(5):902–7. https://doi.org/10.4103/1319-2442.190781.
Guadalupe Vásquez-Mendoza MA, Vargas-Origel A, Del Carmen R-J, Aguilar-Orozco G, Romero-Gutiérrez G. Efficacy and renal toxicity of one daily dose of amikacin versus conventional dosage regime. Amer J Perinatol. 2007;24(2):141–6. https://doi.org/10.1055/s-2006-958159.
Endo A, Hanawa K, Nemoto A, et al. Evaluation of nephrotoxicity and ototoxicity following amikacin administration once daily or every 48 hours in neonates. Medicine. 2022;101(43): e31425. https://doi.org/10.1097/MD.0000000000031425.
Medjebeur Hanna R, Levy M, Bille E, et al. Assessment of the effects of a high amikacin dose on plasma peak concentration in critically ill children. Pediatr Drugs. 2021;23(4):395–401. https://doi.org/10.1007/s40272-021-00456-0.
Sandler ES, Mustafa MM, Tkaczewski I, et al. Use of amphotericin B colloidal dispersion in children. J Pediatr Hematol Oncol. 2000;22(3):242–6. https://doi.org/10.1097/00043426-200005000-00009.
Alexander BD, Wingard JR. Study of renal safety in amphotericin B lipid complex-treated patients. Clin Infect Dis. 2005;40(Suppl. 6):S414–21. https://doi.org/10.1086/429335.
Cetin H, Yalaz M, Akisu M, Hilmioglu S, Metin D, Kultursay N. The efficacy of two different lipid-based amphotericin B in neonatal Candida septicemia. Pediatr Int. 2005;47(6):676–80. https://doi.org/10.1111/j.1442-200x.2005.02135.x.
Ambreen G, Rehman A, Hussain K, et al. Neonatal fluid and electrolytes profile effect on amphotericin B associated nephrotoxicity in neonatal tertiary care unit of Karachi-Pakistan. Expert Opin Drug Saf. 2020;19(9):1209–17. https://doi.org/10.1080/14740338.2020.1781813.
Le J, Adler-Shohet FC, Nguyen C, Lieberman JM. Nephrotoxicity associated with amphotericin B deoxycholate in neonates. Pediatr Infect Dis J. 2009;28(12):1061–3. https://doi.org/10.1097/INF.0b013e3181af6201.
Turcu R, Patterson MJ, Omar S. Influence of sodium intake on amphotericin B-induced nephrotoxicity among extremely premature infants. Pediatr Nephrol. 2009;24(3):497–505. https://doi.org/10.1007/s00467-008-1050-4.
Jeon GW, Koo SH, Lee JH, et al. A comparison of Am Bisome to amphotericin B for treatment of systemic candidiasis in very low birth weight infants. Yonsei Med J. 2007;48(4):619–26. https://doi.org/10.3349/ymj.2007.48.4.619.
Holler B, Omar SA, Farid MD, Patterson MJ. Effects of fluid and electrolyte management on amphotericin B-induced nephrotoxicity among extremely low birth weight infants. Pediatrics. 2004;113(6):e608–16. https://doi.org/10.1542/peds.113.6.e608.
Krepis P, Argyri I, Krepi A, Syrmou A, Spyridis N, Tsolia M. Short-course regimens of liposomal amphotericin B for the treatment of Mediterranean visceral leishmaniasis in children: an 11-year retrospective study at a tertiary care center. Pediatr Infect Dis J. 2017;36(9):849–54. https://doi.org/10.1097/INF.0000000000001602.
Manzoni P, Galletto P, Rizzollo S, et al. Liposomal amphotericin B does not induce nephrotoxicity or renal function impairment in premature neonates. Early Hum Dev. 2012;88(Suppl. 2):S86-91. https://doi.org/10.1016/S0378-3782(12)70024-5.
Kolve H, Ahlke E, Fegeler W, Ritter J, Jürgens H, Groll AH. Safety, tolerance and outcome of treatment with liposomal amphotericin B in paediatric patients with cancer or undergoing haematopoietic stem cell transplantation. J Antimicrob Chemother. 2009;64(2):383–7. https://doi.org/10.1093/jac/dkp196.
McLintock LA, Cook G, Holyoake TL, Jones BL, Kinsey SE, Jackson GH. High loading dose Am Bisome is efficacious and well tolerated in the management of invasive fungal infection in hematology patients. Haematologica. 2007;92(4):572–3. https://doi.org/10.3324/haematol.10531.
Devrim F, Çağlar İ, Acar SO, et al. Evaluation of renal effects of liposomal amphotericin B in children with malignancies with KDIGO and RIFLE criteria. Nephrol Ther. 2021;17(7):507–11. https://doi.org/10.1016/j.nephro.2021.06.007.
Ibrahim SL, Zhang L, Brady TM, Hsu AJ, Cosgrove SE, Tamma PD. Low-dose gentamicin for uncomplicated Enterococcus faecalis bacteremia may be nephrotoxic in children. Clin Infect Dis. 2015;61(7):1119–24. https://doi.org/10.1093/cid/civ461.
Cesaro S, Giacchino M, Locatelli F, et al. Safety and efficacy of a caspofungin-based combination therapy for treatment of proven or probable aspergillosis in pediatric hematological patients. BMC Infect Dis. 2007;7(1):28. https://doi.org/10.1186/1471-2334-7-28.
Maertens JA, Madero L, Reilly AF, et al. A randomized, double-blind, multicenter study of caspofungin versus liposomal amphotericin B for empiric antifungal therapy in pediatric patients with persistent fever and neutropenia. Pediatr Infect Dis J. 2010;29(5):415–20. https://doi.org/10.1097/INF.0b013e3181da2171.
Imburgia TA, Engdahl SR, Pettit RS. Evaluation of the safety of cefepime prolonged infusions in pediatric patients with cystic fibrosis. Pediatr Pulmonol. 2022;57(4):919–25. https://doi.org/10.1002/ppul.25817.
Ganapathi L, Arnold A, Jones S, et al. Use of cidofovir in pediatric patients with adenovirus infection. F1000Res. 2016;5:758. https://doi.org/10.12688/f1000research.8374.2.
Vora SB, Brothers AW, Englund JA. Renal toxicity in pediatric patients receiving cidofovir for the treatment of adenovirus infection. J Pediatr Infect Dis Soc. 2017;6(4):399–402. https://doi.org/10.1093/jpids/pix011.
Caruso Brown AE, Cohen MN, Tong S, et al. Pharmacokinetics and safety of intravenous cidofovir for life-threatening viral infections in pediatric hematopoietic stem cell transplant recipients. Antimicrob Agents Chemother. 2015;59(7):3718–25. https://doi.org/10.1128/AAC.04348-14.
Pérez V, Saénz D, Madriz J, et al. A double-blind study of the efficacy and safety of multiple daily doses of amikacin versus one daily dose for children with perforated appendicitis in Costa Rica. Int J Infect Dis. 2011;15(8):e569–75. https://doi.org/10.1016/j.ijid.2011.04.012.
Ilhan O, Bor M, Ozdemir SA, Akbay S, Ozer EA. Efficacy and safety of intravenous colistin in very low birth weight preterm infants. Pediatr Drugs. 2018;20(5):475–81. https://doi.org/10.1007/s40272-018-0301-5.
Sahbudak Bal Z, Kamit Can F, Yazici P, et al. The evaluation of safety and efficacy of colistin use in pediatric intensive care unit: results from two reference hospitals and review of literature. J Infect Chemother. 2018;24(5):370–5. https://doi.org/10.1016/j.jiac.2017.12.017.
İpek MS, Aktar F, Okur N, Celik M, Ozbek E. Colistin use in critically ill neonates: a case-control study. Pediatr Neonatol. 2017;58(6):490–6. https://doi.org/10.1016/j.pedneo.2016.10.002.
Çağan E, Kıray Baş E, Asker HS. Use of colistin in a neonatal intensive care unit: a cohort study of 65 patients. Med Sci Monit. 2017;23:548–54. https://doi.org/10.12659/MSM.898213.
Kumar PP, Giri SR, Shaikh FAR, Panigrahy N, Chirla D. Safety and efficacy of intravenous colistin in children. Indian Pediatr. 2015;52(2):129–30. https://doi.org/10.1007/s13312-015-0586-1.
Karli A, Paksu MS, Karadag A, et al. Colistin use in pediatric intensive care unit for severe nosocomial infections: experience of an university hospital. Ann Clin Microbiol Antimicrob. 2013;12:32. https://doi.org/10.1186/1476-0711-12-32.
Ozsurekci Y, Aykac K, Cengiz AB, et al. Is colistin effective in the treatment of infections caused by multidrug-resistant (MDR) or extremely drug-resistant (XDR) Gram-negative microorganisms in children? Diagn Microbiol Infect Dis. 2016;85(2):233–8. https://doi.org/10.1016/j.diagmicrobio.2016.02.017.
Alan S, Yildiz D, Erdeve O, et al. Efficacy and safety of intravenous colistin in preterm infants with nosocomial sepsis caused by Acinetobacter baumannii. Am J Perinatol. 2014;31(12):1079–86. https://doi.org/10.1055/s-0034-1371361.
Tamma PD, Newland JG, Pannaraj PS, et al. The use of intravenous colistin among children in the United States: results from a multicenter, case series. Pediatr Infect Dis J. 2013;32(1):17–22. https://doi.org/10.1097/INF.0b013e3182703790.
Paksu MS, Paksu S, Karadag A, et al. Old agent, new experience: colistin use in the paediatric intensive care unit: a multicentre study. Int J Antimicrob Agents. 2012;40(2):140–4. https://doi.org/10.1016/j.ijantimicag.2012.04.010.
Fatehi S, Eshaghi H, Sharifzadeh M, et al. A randomized clinical trial evaluating the efficacy of colistin loading dose in critically ill children. J Res Pharm Pract. 2019;8(4):196. https://doi.org/10.4103/jrpp.JRPP_19_68.
Ambreen G, Salat MS, Hussain K, et al. Efficacy of colistin in multidrug-resistant neonatal sepsis: experience from a tertiary care center in Karachi. Pakistan Arch Dis Child. 2020;105(9):830–6. https://doi.org/10.1136/archdischild-2019-318067.
Wacharachaisurapol N, Phasomsap C, Sukkummee W, et al. Greater optimisation of pharmacokinetic/pharmacodynamic parameters through a loading dose of intravenous colistin in paediatric patients. Int J Antimicrob Agents. 2020;55(6): 105940. https://doi.org/10.1016/j.ijantimicag.2020.105940.
Aksoy GK, Özkan SEÖ, Tezel G, Dayar GT, Köşker M, Doğan ÇS. Assesment of colistin related side effects in premature neonates. Turk J Pediatr. 2020;62(5):795. https://doi.org/10.24953/turkjped.2020.05.011.
Wacharachaisurapol N, Kawichai S, Chanakul A, Puthanakit T. No increased acute kidney injury rate through giving an intravenous colistin loading dose in pediatric patients. Int J Infect Dis. 2021;106:91–7. https://doi.org/10.1016/j.ijid.2021.03.059.
Meysam S, Khosravi Z, Rashti R, et al. Colistin induced acute kidney injury in critically ill children: a prospective study utilizing RIFLE criteria. DARU J Pharm Sci. 2022;30(1):11–5. https://doi.org/10.1007/s40199-021-00421-9.
Lee J, Kim HS, Shin SH, et al. Efficacy and safety of fluconazole prophylaxis in extremely low birth weight infants: multicenter pre-post cohort study. BMC Pediatr. 2016;16:67. https://doi.org/10.1186/s12887-016-0605-y.
Vora SB, Brothers AW, Waghmare A, Englund JA. Antiviral combination therapy for cytomegalovirus infection in high-risk infants. Antivir Ther. 2018;23(6):505–11. https://doi.org/10.3851/IMP3238.
Carapetis JR, Jaquiery AL, Buttery JP, et al. Randomized, controlled trial comparing once daily and three times daily gentamicin in children with urinary tract infections. Pediatr Infect Dis J. 2001;20(3):240–6. https://doi.org/10.1097/00006454-200103000-00004.
Constance JE, Reith D, Ward RM, et al. Risk of nonsteroidal anti-inflammatory drug-associated renal dysfunction among neonates diagnosed with patent ductus arteriosus and treated with gentamicin. J Perinatol. 2017;37(10):1093–102. https://doi.org/10.1038/jp.2017.80.
Lau L, Al-Ismaili Z, Harel-Sterling M, et al. Serum cystatin C for acute kidney injury evaluation in children treated with aminoglycosides. Pediatr Nephrol. 2017;32(1):163–71. https://doi.org/10.1007/s00467-016-3450-1.
Constance JE, Balch AH, Stockmann C, et al. A propensity-matched cohort study of vancomycin-associated nephrotoxicity in neonates. Arch Dis Child Fetal Neonatal Ed. 2016;101(3):F236–43. https://doi.org/10.1136/archdischild-2015-308459.
Jansen D, Peters E, Heemskerk S, et al. Tubular injury biomarkers to detect gentamicin-induced acute kidney injury in the neonatal intensive care unit. Am J Perinatol. 2016;33(2):180–7. https://doi.org/10.1055/s-0035-1563714.
McWilliam SJ, Antoine DJ, Sabbisetti V, et al. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study. PLoS ONE. 2012;7(8): e43809. https://doi.org/10.1371/journal.pone.0043809.
Martínková J, Pokorná P, Záhora J, et al. Tolerability and outcomes of kinetically guided therapy with gentamicin in critically ill neonates during the first week of life: an open-label, prospective study. Clin Ther. 2010;32(14):2400–14. https://doi.org/10.1016/j.clinthera.2011.01.013.
Serane TV, Zengeya S, Penford G, Cooke J, Khanna G, McGregor-Colman E. Once daily dose gentamicin in neonates: is our dosing correct? Acta Paediatr. 2009;98(7):1100–5. https://doi.org/10.1111/j.1651-2227.2009.01297.x.
Zappitelli M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011;26(1):144–50. https://doi.org/10.1093/ndt/gfq375.
Khan AM, Ahmed T, Alam NH, Chowdhury AK, Fuchs GJ. Extended-interval gentamicin administration in malnourished children. J Trop Pediatr. 2006;52(3):179–84. https://doi.org/10.1093/tropej/fmi085.
Chong CY, Tan ASL, Ng W, Tan-Kendrick A, Balakrishnan A, Chao SM. Treatment of urinary tract infection with gentamicin once or three times daily. Acta Paediatr. 2003;92(3):291–6.
Sridharan K, Al DA. Clinical audit of gentamicin use by Bayesian pharmacokinetic approach in critically ill children. J Infect Chemother. 2020;26(6):540–8. https://doi.org/10.1016/j.jiac.2020.01.007.
Sridharan K, Al Jufairi M, Al Segai O, et al. Biomarkers in neonates receiving potential nephrotoxic drugs. Eur Rev Med Pharmacol Sci. 2021;25(22):7078–88. https://doi.org/10.26355/eurrev_202111_27260.
Uijtendaal EV, Rademaker CM, Schobben AF, et al. Once-daily versus multiple-daily gentamicin in infants and children. Ther Drug Monit. 2001;23(5):506–13. https://doi.org/10.1097/00007691-200110000-00002.
Polat M, Kara SS. Once-daily intramuscular amikacin for outpatient treatment of lower urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli in children. Infect Drug Resist. 2017;10:393–9. https://doi.org/10.2147/IDR.S148703.
Bayram N, Düzgöl M, Kara A, Özdemir FM, Devrim İ. Linezolid-related adverse effects in clinical practice in children. Arch Argent Pediatr. 2017;115(5):470–5. https://doi.org/10.5546/aap.2017.eng.470.
Shabaan AE, Nour I, Elsayed Eldegla H, Nasef N, Shouman B, Abdel-Hady H. Conventional versus prolonged infusion of meropenem in neonates with Gram-negative late-onset sepsis: a randomized controlled trial. Pediatr Infect Dis J. 2017;36(4):358–63. https://doi.org/10.1097/INF.0000000000001445.
Yoshikawa K, Nakazawa Y, Katsuyama Y, et al. Safety, tolerability, and feasibility of antifungal prophylaxis with micafungin at 2 mg/kg daily in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Infection. 2014;42(4):639–47. https://doi.org/10.1007/s15010-014-0601-9.
Arrieta AC, Maddison P, Groll AH. Safety of micafungin in pediatric clinical trials. Pediatr Infect Dis J. 2011;30(6):e97-102. https://doi.org/10.1097/INF.0b013e3182127eaf.
Wang PL, Liu P, Zhang QW, et al. Population pharmacokinetics and clinical outcomes of polymyxin B in paediatric patients with multidrug-resistant Gram-negative bacterial infections. J Antimicrob Chemother. 2022;77(11):3000–8. https://doi.org/10.1093/jac/dkac265.
Barco-Cabrera C, Reina YA, Dávalos DM, et al. Use of polymyxins for carbapenem-resistant infections in children and adolescents. JAC Antimicrob Resist. 2022;4(3):dlac073. https://doi.org/10.1093/jacamr/dlac073.
Jia X, Yin Z, Zhang W, Guo C, Du S, Zhang X. Effectiveness and nephrotoxicity of intravenous polymyxin B in carbapenem-resistant Gram-negative bacterial infections among Chinese children. Front Pharmacol. 2022;13: 902054. https://doi.org/10.3389/fphar.2022.902054.
Yamada T, Kubota T, Nakamura M, et al. Evaluation of teicoplanin concentrations and safety analysis in neonates. Int J Antimicrob Agents. 2014;44(5):458–62. https://doi.org/10.1016/j.ijantimicag.2014.07.005.
Yamada T, Kubota T, Yonezawa M, et al. Evaluation of teicoplanin trough values after the recommended loading dose in children with associated safety analysis. Pediatr Infect Dis J. 2017;36(4):398–400. https://doi.org/10.1097/INF.0000000000001456.
Sidi V, Roilides E, Bibashi E, Gompakis N, Tsakiri A, Koliouskas D. Comparison of efficacy and safety of teicoplanin and vancomycin in children with antineoplastic therapy-associated febrile neutropenia and gram-positive bacteremia. J Chemother. 2000;12(4):326–31. https://doi.org/10.1179/joc.2000.12.4.326.
Ju M, Zheng M, Yuan J, Lin D, Qian Y. Prevalence and risk factors of trimethoprim/sulfamethoxazole-related acute kidney injury in pediatric patients: an observational study from a public database. Transl Pediatr. 2022;11(8):1285–91. https://doi.org/10.21037/tp-21-600.
Sierra CM, Tran Y, Oana L, Bahjri K. Renal impairment associated with trimethoprim-sulfamethoxazole use in the pediatric population. J Pediatr Pharmacol Ther. 2022;27(7):663–8. https://doi.org/10.5863/1551-6776-27.7.663.
Arends A, Pettit R. Safety of extended interval tobramycin in cystic fibrosis patients less an 6 years old. J Pediatr Pharmacol Ther. 2018;23(2):152–8. https://doi.org/10.5863/1551-6776-23.2.152.
Imani S, Fitzgerald DA, Robinson PD, Selvadurai H, Sandaradura I, Lai T. Personalized tobramycin dosing in children with cystic fibrosis: a comparative clinical evaluation of log-linear and Bayesian methods. J Antimicrob Chemother. 2022;77(12):3358–66. https://doi.org/10.1093/jac/dkac324.
Hayes M, Boge CLK, Sharova A, et al. Antiviral toxicities in pediatric solid organ transplant recipients. Am J Transpl. 2022;22(12):3012–20. https://doi.org/10.1111/ajt.17171.
LeCleir LK, Pettit RS. Piperacillin-tazobactam versus cefepime incidence of acute kidney injury in combination with vancomycin and tobramycin in pediatric cystic fibrosis patients. Pediatr Pulmonol. 2017;52(8):1000–5. https://doi.org/10.1002/ppul.23718.
Cook KM, Gillon J, Grisso AG, et al. Incidence of nephrotoxicity among pediatric patients receiving vancomycin with either piperacillin-tazobactam or cefepime: a cohort study. J Pediatr Infect Dis Soc. 2019;8(3):221–7. https://doi.org/10.1093/jpids/piy030.
Holsen MR, Meaney CJ, Hassinger AB, Fusco NM. Increased risk of acute kidney injury in critically ill children treated with vancomycin and piperacillin/tazobactam. Pediatr Crit Care Med. 2017;18(12):e585–91. https://doi.org/10.1097/PCC.0000000000001335.
Joyce EL, Kane-Gill SL, Priyanka P, Fuhrman DY, Kellum JA. Piperacillin/tazobactam and antibiotic-associated acute kidney injury in critically ill children. J Am Soc Nephrol. 2019;30(11):2243–51. https://doi.org/10.1681/ASN.2018121223.
Alqurashi R, Batwa M, Alghamdi B, et al. Acute kidney injury in pediatric patients treated with vancomycin and piperacillin-tazobactam versus vancomycin and cefotaxime: a single-center study. Cureus. 2020;12(1): e6805. https://doi.org/10.7759/cureus.6805.
Bartlett JW, Gillon J, Hale J, Jimenez-Truque N, Banerjee R. Incidence of acute kidney injury among infants in the neonatal intensive care unit receiving vancomycin with either piperacillin/tazobactam or cefepime. J Pediatr Pharmacol Ther. 2020;25(6):521–7. https://doi.org/10.5863/1551-6776-25.6.521.
Al-Jebawi Y, Karalic K, Shekhawat P, Mhanna MJ. The concomitant use of vancomycin and piperacillin-tazobactam is associated with acute kidney injury (AKI) in extremely low birth weight infants (ELBW). J Neonatal Perinatal Med. 2022;15(2):303–9. https://doi.org/10.3233/NPM-210866.
Abouelkheir M, Alsubaie S. Pediatric acute kidney injury induced by concomitant vancomycin and piperacillin-tazobactam. Pediatr Int. 2018;60(2):136–41. https://doi.org/10.1111/ped.13463.
Zhang H, Wang Y, Gao P, et al. Pharmacokinetic characteristics and clinical outcomes of vancomycin in young children with various degrees of renal function. J Clin Pharmacol. 2016;56(6):740–8. https://doi.org/10.1002/jcph.653.
Sun Y, Huskey RL, Tang L, et al. Adverse effects of intravenous vancomycin-based prophylaxis during therapy for pediatric acute myeloid leukemia. Antimicrob Agents Chemother. 2018;62(3):e01838-e1917. https://doi.org/10.1128/AAC.01838-17.
Knoderer CA, Gritzman AL, Nichols KR, Wilson AC. Late-occurring vancomycin-associated acute kidney injury in children receiving prolonged therapy. Ann Pharmacother. 2015;49(10):1113–9. https://doi.org/10.1177/1060028015594190.
Moffett BS, Hilvers PS, Dinh K, Arikan AA, Checchia P, Bronicki R. Vancomycin-associated acute kidney injury in pediatric cardiac intensive care patients. Congenit Heart Dis. 2015;10(1):E6-10. https://doi.org/10.1111/chd.12187.
Sinclair EA, Yenokyan G, McMunn A, Fadrowski JJ, Milstone AM, Lee CKK. Factors associated with acute kidney injury in children receiving vancomycin. Ann Pharmacother. 2014;48(12):1555–62. https://doi.org/10.1177/1060028014549185.
Linder N, Lubin D, Hernandez A, Amit L, Ashkenazi S. Duration of vancomycin treatment for coagulase-negative Staphylococcus sepsis in very low birth weight infants. Br J Clin Pharmacol. 2013;76(1):58–64. https://doi.org/10.1111/bcp.12053.
Bhargava V, Malloy M, Fonseca R. The association between vancomycin trough concentrations and acute kidney injury in the neonatal intensive care unit. BMC Pediatr. 2017;17(1):50. https://doi.org/10.1186/s12887-017-0777-0.
Wei WX, Qin XL, Cheng DH, Lu H, Liu TT. Retrospective analysis of vancomycin treatment outcomes in Chinese paediatric patients with suspected Gram-positive infection. J Clin Pharm Ther. 2016;41(6):650–6. https://doi.org/10.1111/jcpt.12437.
Seixas GTF, Araujo OR, Silva DCB, Arduini RG, Petrilli AS. Vancomycin therapeutic targets and nephrotoxicity in critically ill children with cancer. J Pediatr Hematol Oncol. 2016;38(2):e56-62. https://doi.org/10.1097/MPH.0000000000000470.
Totapally BR, Machado J, Lee H, Paredes A, Raszynski A. Acute kidney injury during vancomycin therapy in critically ill children. Pharmacotherapy. 2013;33(6):598–602. https://doi.org/10.1002/phar.1259.
McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;158(3):422–6. https://doi.org/10.1016/j.jpeds.2010.08.019.
Quach HT, Esbenshade AJ, Zhao Z, Banerjee R. Incidence of acute kidney injury among pediatric hematology/oncology patients receiving vancomycin in combination with piperacillin/tazobactam or cefepime. Pediatr Blood Cancer. 2019;66(7): e27750. https://doi.org/10.1002/pbc.27750.
Reilly AM, Ding MX, Rower JE, Kiser TH. The effectiveness of a vancomycin dosing guideline in the neonatal intensive care unit for achieving goal therapeutic trough concentrations. J Clin Pharmacol. 2019;59(7):997–1005. https://doi.org/10.1002/jcph.1392.
Zhang T, Cheng H, Li Y, et al. Paediatric acute kidney injury induced by vancomycin monotherapy versus combined vancomycin and meropenem. J Clin Pharm Ther. 2019;44(3):440–6. https://doi.org/10.1111/jcpt.12806.
Hing WC, Bek SJ, Lin RTP, Li SC. A retrospective drug utilization evaluation of vancomycin usage in paediatric patients. J Clin Pharm Ther. 2004;29(4):359–65. https://doi.org/10.1111/j.1365-2710.2004.00571.x.
Hurst AL, Baumgartner C, MacBrayne CE, Child J. Experience with continuous infusion vancomycin dosing in a large pediatric hospital. J Pediatr Infect Dis Soc. 2019;8(2):174–9. https://doi.org/10.1093/jpids/piy032.
Fitzgerald JC, Zane NR, Himebauch AS, et al. Vancomycin prescribing and therapeutic drug monitoring in children with and without acute kidney injury after cardiac arrest. Pediatr Drugs. 2019;21(2):107–12. https://doi.org/10.1007/s40272-019-00328-8.
Woldu H, Guglielmo BJ. Incidence and risk factors for vancomycin nephrotoxicity in acutely ill pediatric patients. J Pharm Technol. 2018;34(1):9–16. https://doi.org/10.1177/8755122517747088.
Morales Junior R, Juodinis VD, Santos ICPDF, et al. Vancomycin area under the curve-guided monitoring in pediatric patients. Rev Bras Ter Intensiva. 2022;34(1):147–53. https://doi.org/10.5935/0103-507X.20220009-en.
Hussain K, Salat MS, Rauf S, et al. Practical approaches to improve vancomycin-related patient outcomes in pediatrics- an alternative strategy when AUC/MIC is not feasible. BMC Pharmacol Toxicol. 2022;23(1):64. https://doi.org/10.1186/s40360-022-00606-1.
Mitchell B, Kormelink L, Kuhn R, Schadler A, Autry E. Retrospective review of vancomycin monitoring via trough only versus two-point estimated area under the curve in pediatric and adult patients with cystic fibrosis. Pediatr Pulmonol. 2023;58(1):239–45. https://doi.org/10.1002/ppul.26190.
Sridharan K, Al-Daylami A, Ajjawi R, Ajooz HAA. Vancomycin use in a paediatric intensive care unit of a tertiary care hospital. Pediatr Drugs. 2019;21(4):303–12. https://doi.org/10.1007/s40272-019-00343-9.
Feiten HDS, Okumura LM, Martinbiancho JK, et al. Vancomycin-associated nephrotoxicity and risk factors in critically ill children without preexisting renal injury. Pediatr Infect Dis J. 2019;38(9):934–8. https://doi.org/10.1097/INF.0000000000002391.
Olson J, Hersh AL, Sorensen J, Zobell J, Anderson C, Thorell EA. Intravenous vancomycin therapeutic drug monitoring in children: evaluation of a pharmacy-driven protocol and collaborative practice agreement. J Pediatr Infect Dis Soc. 2020;9(3):334–41. https://doi.org/10.1093/jpids/piz036.
Sosnin N, Curtis N, Cranswick N, Chiletti R, Gwee A. Vancomycin is commonly under-dosed in critically ill children and neonates. Br J Clin Pharmacol. 2019;85(11):2591–8. https://doi.org/10.1111/bcp.14084.
Zhang H, Gao P, Wang Y, et al. Baseline kidney function is associated with vancomycin-induced acute kidney injury in children: a prospective nested case-control study. Pediatr Nephrol. 2021;36(5):1299–306. https://doi.org/10.1007/s00467-020-04820-z.
Tang Z, Guan J, Li J, et al. Determination of vancomycin exposure target and individualised dosing recommendations for neonates: model-informed precision dosing. Int J Antimicrob Agents. 2021;57(3): 106300. https://doi.org/10.1016/j.ijantimicag.2021.106300.
Kwak S, Kim JY, Cho H. Vancomycin-induced nephrotoxicity in non-intensive care unit pediatric patients. Sci Rep. 2021;11(1):20681. https://doi.org/10.1038/s41598-021-00214-9.
Hambrick HR, Greco KF, Weller E, Ganapathi L, Lehmann LE, Sandora TJ. Impact of decreasing vancomycin exposure on acute kidney injury in stem cell transplant recipients. Infect Control Hosp Epidemiol. 2022;43(10):1375–81. https://doi.org/10.1017/ice.2021.454.
Dawoud TH, Khan N, Afzal U, Varghese N, Rahmani A, Abu-Sa’da O. Assessment of initial vancomycin trough levels and risk factors of vancomycin-induced nephrotoxicity in neonates. Eur J Hosp Pharm. 2022;29(1):44–9. https://doi.org/10.1136/ejhpharm-2019-002181.
Chen Q, Wan J, Shen W, et al. Optimal exposure targets for vancomycin in the treatment of neonatal coagulase-negative Staphylococcus infection: a retrospective study based on electronic medical records. Pediatr Neonatol. 2022;63(3):247–54. https://doi.org/10.1016/j.pedneo.2021.11.010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Ibram Mikhail is supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number T32GM008562. Kevin J. Downes is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD091365.
Conflicts of interest/competing interests
Torsten Joerger, Molly Hayes, Connor Stinson, Ibram Mikhail, and Kevin J. Downes have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
KJD conceptualized and designed the study. TJ, MH, and KJD screened articles for inclusion and exclusion criteria. TJ, MH, KJD, CS, and IM extracted rates of nephrotoxicity from selected articles and IM compiled summarizing information into Table 1 of the ESM. TJ and KJD analyzed the data and wrote the manuscript. All authors reviewed, provided critical feedback, and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joerger, T., Hayes, M., Stinson, C. et al. Incidence of Antimicrobial-Associated Acute Kidney Injury in Children: A Structured Review. Pediatr Drugs 26, 59–70 (2024). https://doi.org/10.1007/s40272-023-00607-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00607-5