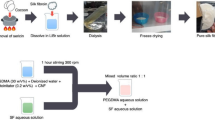
Abstract
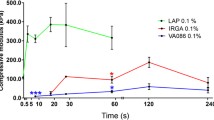
The effect of riboflavin-induced collagen photocrosslinking on the scaffold printing accuracy, namely on the area of formed niches and filament thickness, as well as on the degradation time and biocompatibility of scaffolds, has been studied. The thickness of the filaments in the riboflavin-modified scaffolds was 13–29% less and the niche area was 23–40% larger than in the control group without riboflavin. The riboflavin addition reduced weight loss and increased the scaffold degradation time in the buffer solution and collagenase solution by 1.2–1.3, and 1.4–2.0 times, respectively, depending on the time of exposure to UV light. The existence of an optimal illumination interval was established. Cultivation of mesenchymal stem cells on a riboflavin-crosslinked scaffold for a week did not lead to a decrease in their viability and proliferation rate.



Similar content being viewed by others
Notes
A counter stroke is printed to prepare the device for smooth filament extrusion.
REFERENCES
Meyer, M., Processing of collagen based biomaterials and the resulting materials properties, Biomed. Eng. Online, 2019, vol. 18, no. 1. https://doi.org/10.1186/s12938-019-0647-0
Jafari, H., Lista, A., Siekapen, M., Ghaffari-Bohlouli, P., Nie, L., Alimoradi, H., and Shavandi, A., Fish collagen: extraction, characterization, and applications for biomaterials engineering, Polymers (Basel), 2020, vol. 12, no. 10, p. 2230. https://doi.org/10.3390/polym12102230
Tytgat, L., Dobos, A., Markovic, M., Van Damme, L., Van Hoorick, J., Bray, F., Thienpont, H., Ottevaere, H., Dubruel, P., Ovsianikov, A., and Van Vlierberghe, S., High-resolution 3D bioprinting of photo-cross-linkable recombinant collagen to serve tissue engineering applications, Biomacromolecules, 2020, vol. 21, no. 10, pp. 3997–4007. https://doi.org/10.1021/acs.biomac.0c00386
Werkmeister, J.A. and Ramshaw, J.A.M., Recombinant protein scaffolds for tissue engineering, Biomed. Mater., 2012, vol. 7, p. 012002. https://doi.org/10.1088/1748-6041/7/1/012002
Koide, T., Designed triple-helical peptides as tools for collagen biochemistry and matrix engineering, Proc. R. Soc. London, Ser. B., 2007, vol. 362, no. 1484, pp. 1281–1291. https://doi.org/10.1098/rstb.2007.2115
Yang, X., Lu, Z., Wu, H., Li, W., Zheng, L., and Zhao, J., Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering, Mater. Sci. Eng. C Mater. Biol. Appl., 2018, vol. 83, pp. 195–201. https://doi.org/10.1016/j.msec.2017.09.002
Zhang, Y., Zhou, D., Chen, J., Zhang, X., Li, X., Zhao, W., and Xu, T., Biomaterials based on marine resources for 3D bioprinting applications, Mar. Drugs, 2019, vol. 17, p. 555. https://doi.org/10.3390/md17100555
Chan, B.P. and So, K.F., Photochemical crosslinking improves the physicochemical properties of collagen scaffolds, J. Biomed. Mater. Res. Part A, 2005, vol. 75, no. 3, pp. 689–701. https://doi.org/10.1002/jbm.a.30469
Rich, H., Odlyha, M., Cheema, U., Mudera, V., and Bozec, L., Effects of photochemical riboflavin-mediated crosslinks on the physical properties of collagen constructs and fibrils, J. Mater. Sci. Mater. Med., 2014, vol. 25, no. 1, pp. 11–21. https://doi.org/10.1007/s10856-013-5038-7
Kamimura, W., Koyama, H., Miyata, T., and Takato, T., Sugar-based crosslinker forms a stable atelocollagen hydrogel that is a favorable microenvironment for 3D cell culture, J. Biomed. Mater. Res. Part A, 2014, vol. 102, pp. 4309–4316. https://doi.org/10.1002/jbm.a.35106
Bi, L., Cao, Z., Hu, Y., Song, Y., Yu, L., Yang, B., Mu, J., Huang, Z., and Han, Y., Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering, J. Mater. Sci. Mater. Med., 2011, vol. 22, no. 1, pp. 51–62. https://doi.org/10.1007/s10856-010-4177-3
Diamantides, N., Wang, L., Pruiksma, T., Siemiatkoski, J., Dugopolski, C., Shortkroff, S., Kennedy, S., and Bonassar, L.J., Correlating rheological properties and printability of collagen bioinks: the effects of riboflavin photocrosslinking and pH, Biofabrication, 2017, vol. 9, no. 3, p. 034102. https://doi.org/10.1088/1758-5090/aa780f
Orban, J.M., Wilson, L.B., Kofroth, J.A., El-Kurdi, M.S., Maul, T.M., and Vorp, D.A., Crosslinking of collagen gels by transglutaminase, J. Biomed. Mater. Res. A, 2004, vol. 68, no. 4, pp. 756–762. https://doi.org/10.1002/jbm.a.20110
Brown, J.H., Das, P., DiVito, M.D., Ivancic, D., Tan, L.P., and Wertheim, J.A., Nanofibrous PLGA electrospun scaffolds modified with type I collagen influence hepatocyte function and support viability in vitro, Acta Biomater., 2018, vol. 73, pp. 217–227. https://doi.org/10.1016/j.actbio.2018.02.009
Jiang, J.P., Liu, X.Y., Zhao, F., Zhu, X., Li, X.Y., Niu, X.G., Yao, Z.T., Dai, C., Xu, H.Y., Ma, K., Chen, X.Y., and Zhang, S., Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury, Neural Regen. Res., 2020, vol. 15, no. 5, pp. 959–968. https://doi.org/10.4103/1673-5374.268974
Peng, Y.Y., Glattauer, V., and Ramshaw, J.A.M., Stabilisation of collagen sponges by glutaraldehyde vapour crosslinking, Int. J. Biomater., 2017, vol. 2017, p. 8947823. https://doi.org/10.1155/2017/8947823
Chandran, P.L., Paik, D.C., and Holmes, J.W., Structural mechanism for alteration of collagen gel mechanics by glutaraldehyde crosslinking, Connect Tissue Res., 2012, vol. 53, no. 4, pp. 285–297. https://doi.org/10.3109/03008207.2011.640760
Kamimura, W., Koyama, H., Miyata, T., and Takato, T., Sugar-based crosslinker forms a stable atelocollagen hydrogel that is a favorable microenvironment for 3D cell culture, J. Biomed. Mater. Res. Part A., 2014, vol. 102, pp. 4309–4316. https://doi.org/10.1002/jbm.a.35106
Yeo, M.G. and Kim, G.H., A cell-printing approach for obtaining hASC-laden scaffolds by using a collagen/polyphenol bioink, Biofabrication, 2017, vol. 9, no. 2, p. 025004. https://doi.org/10.1088/1758-5090/aa699728402968
Jafari-Sabet, M., Nasiri, H., and Ataee, R., The effect of crosslinking agents and collagen concentrations on properties of collagen scaffolds, J. Arch. Mil. Med., 2016, vol. 4. https://doi.org/10.5812/JAMM.42367
Chen, R., Ho, H., and Sheu, T., Characterization of collagen matrices crosslinked using microbial transglutaminase, Biomaterials, 2005, vol. 26, no. 20, pp. 4229–4235. https://doi.org/10.1016/j.biomaterials.2004.11.012
Nakada, A., Shigeno, K., Sato, T., Hatayama, T., Wakatsuki, M., and Nakamura, T., Optimal dehydrothermal processing conditions to improve biocompatibility and durability of a weakly denatured collagen scaffold, J. Biomed. Mater. Res. Part B Appl. Biomater., 2017, vol. 105, pp. 2301–2307. https://doi.org/10.1002/jbm.b.33766
Pawelec, K.M., Confalonieri, D., Ehlicke, F., van Boxtel, H.A., Walles, H., and Kluijtmans, S.G.J.M., Osteogenesis and mineralization of mesenchymal stem cells in collagen type I-based recombinant peptide scaffolds, J. Biomed. Mater. Res. Part A., 2017, vol. 105, no. 7, pp. 1856–1866. https://doi.org/10.1002/jbm.a.36049
Weadock, K., Miller, E., Keuffel, E., and Dunn, M., Effect of physical crosslinking methods on collagen-fiber durability in proteolytic solutions, J. Biomed. Mater. Res., 1996, vol. 32, pp. 221–226. https://doi.org/10.1002/(SICI)1097-4636(199610)-32:2<221::AID-JBM11>3.0.CO;2-M
Choi, G. and Cha, H.J., Recent advances in the development of nature-derived photocrosslinkable biomaterials for 3D printing in tissue engineering, Biomater. Res., 2019, vol. 23, p. 18. https://doi.org/10.1186/s40824-019-0168-8
Ionita, G. and Matei, I., Application of riboflavin photochemical properties in hydrogel synthesis, Biophys. Chem., Adv. Appl., 2019. https://doi.org/10.5772/intechopen.88855
Tonndorf, R., Gossla, E., Aibibu, D., Lindner, M., Gelinsky, M., and Cherif, C., Wet spinning and ribof lavin crosslinking of collagen type I/III filaments, Biomed Mater., 2018, vol. 14, no. 1, p. 015007. https://doi.org/10.1088/1748-605X/aaebda
Raiskup, F. and Spoerl, E., Corneal crosslinking with ribof lavin and ultraviolet A. I, Principles Ocul. Surf., 2013, vol. 11, no. 2, pp. 65–74. https://doi.org/10.1016/j.jtos.2013.01.002
Maier, P., Reinhard, T., and Kohlhaas, M., Corneal collagen cross-linking in the stabilization of keratoconus, Dtsch. Arztebl. Int., 2019, vol. 116, no. 11, pp. 184–190. https://doi.org/10.3238/arztebl.2019.0184
Daood, U., Heng, C.S., Neo Chiew Lian, J., and Fawzy, A.S., In vitro analysis of ribof lavin-modified, experimental, two-step etch-and-rinse dentin adhesive: Fourier transform infrared spectroscopy and micro-Raman studies, Int. J. Oral. Sci., 2015, vol. 7, no. 2, pp. 110–124. https://doi.org/10.1038/ijos.2014.49
Grunert P., Borde B.H., Towne S.B., Moriguchi Y., Hudson K.D., Bonassar L.J., Hartl R. Ribof lavin crosslinked high-density collagen gel for the repair of annular defects in intervertebral discs: an in vivo study, Acta Biomater., 2015, vol. 26, pp. 215–224. https://doi.org/10.1016/j.actbio.2015.06.006
Choi, B., Kim, S., Lin, B., Bezouglaia, O., Kim, J., Evseenko, D., Aghaloo, T., and Lee, M., Visible-light-initiated hydrogels preserving cartilage extracellular signaling for inducing chondrogenesis of mesenchymal stem cells, Acta Biomater., 2015, vol. 12C, pp. 30–41. https://doi.org/10.1016/j.actbio.2014.10.013
Heo, J., Koh, R.H., Shim, W., Kim, H.D., Yim, H.G., and Hwang, N.S., Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering, Drug Delivery Transl Res., 2016, vol. 6, no. 2, pp. 148–158. https://doi.org/10.1007/s13346-015-0224-4
Cheema, U., Rong, Z., Kirresh, O., Macrobert, A.J., Vadgama, P., and Brown, R.A., Oxygen diffusion through collagen scaffolds at defined densities: implications for cell survival in tissue models, J. Tissue Eng. Regen. Med., 2012, vol. 6, no. 1, pp. 77–84. https://doi.org/10.1002/term.402
Smyshlyaev, I.A., Gil’fanov, S.I., Kopylov, V.A., Gil’mutdinov, R.G., Pulin, A.A., Korsakov, I.N., Gil’mutdinova, I.R., Petrikina, A.P., Eremin, P.S., Kryuchkova, O.V., Abel’tsev, V.P., Zagorodny, N.V., Zorin, V.L., Vasil’ev, V.S., Pupynin, D.Yu., and Eremin, I.I., Evaluation of safety and efficacy of intra-articular injection of stromal vascular adipose tissue fraction for the treatment of gonarthrosis: interim results of a clinical trial, Travmatol. Ortoped. Rossii, 2017, vol. 23, no. 3, pp. 17–31.
Samchuk, D.P., Luk’yanova, E.N., Eremin, I.I., Zorin, V.L., Zorina, A.I., Grinakovskaya, O.S., Korsakov, I.N., Deev, R.V., Gil’mutdinova, I.R., Lazareva, N.L., Eremin, P.S., Petrikina, A.P., Gomzyakov, A.E., Timashkov, D.A., Vit’ko, N.K., Kotenko, K.V., Kopnin, P.B., and Pulin, A.A., Expression of myogenesis genes by gingival mucosal cells, Geny Kletki, 2015, vol. 10, no. 4, pp. 68–77.
Lu, M., Song, X., Yang, M., Kong, W., and Zhu, J., Combined effects of glutaraldehyde and ribof lavin/uv365 on the self-assembly of type I collagen molecules observed with atomic force microscopy, Int. J. Food Properties, 2018, vol. 21, no. 1, pp. 2181–2192. https://doi.org/10.1080/10942912.2018.1510837
Ibusuki, S., Halbesma, G.J., Randolph, M.A., Redmond, R.W., Kochevar, I.E., and Gill, T.J., Photochemically cross-linked collagen gels as three-dimensional scaffolds for tissue engineering, Tissue Eng., 2007, vol. 13, no. 8, pp. 1995–2001. https://doi.org/10.1089/ten.2006.0153
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct thisparticular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
Additional information
Translated by I. Gordon
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: EDTA, ethylenediamine tetraacetate; MSCs, mesenchymal stem cells; PBS, phosphate-buffered saline; PLA, polylactide; UV, ultraviolet.
Rights and permissions
About this article
Cite this article
Arguchinskaya, N.V., Beketov, E.E., Isaeva, E.V. et al. Riboflavin-Induced Photocrosslinking of Highly Concentrated Collagen: Printing Accuracy, Degradation Time, and Cytocompatibility. Appl Biochem Microbiol 59, 1062–1070 (2023). https://doi.org/10.1134/S0003683823080033
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823080033