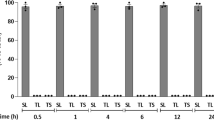
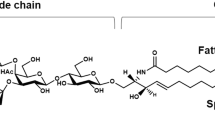
Abstract
Since the 1980s, it has been known that the administration of ganglioside GM1 to cultured cells induced or enhanced neuronal differentiation. GM1 mechanism of action relies on its direct interaction and subsequent activation of the membrane tyrosine kinase receptor, TrkA, which naturally serves as NGF receptor. This process is mediated by the sole oligosaccharide portion of GM1, the pentasaccharide β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-β-Glc. Here we detailed the minimum structural requirements of the oligosaccharide portion of GM1 for mediating the TrkA dependent neuritogenic processing. By in vitro and in silico biochemical approaches, we demonstrated that the minimal portion of GM1 required for the TrkA activation is the inner core of the ganglioside’s oligosaccharide β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal. The addition of a sialic acid residue at position 3 of the outer galactose of the GM1 oligosaccharide, which forms the oligosaccharide of GD1a, prevented the interaction with TrkA and the resulting neuritogenesis. On the contrary, the addition of a fucose residue at position 2 of the outer galactose, forming the Fucosyl-GM1 oligosaccharide, did not prevent the TrkA-mediated neuritogenesis.






Similar content being viewed by others
Data availability statement
The data presented in this study are available upon reasonable request to the corresponding author.
Abbreviations
- Asialo-GM1:
-
Gg4Cer, β-Gal-(1-3)-β-GalNAc-(1-4)-β-Gal-(1-4)-β-Glc-(1-1)-Cer
- Asialo-OligoGM1:
-
Gg4, β-Gal-(1-3)-β-GalNAc-(1-4)-β-Gal-(1-4)-Glc
- BSA:
-
Bovine serum albumin
- CTRL:
-
Control
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DTT:
-
1,4-Dithiothreitol
- ESI:
-
Electrospray ionization process
- FBS:
-
Fetal bovine serum
- Fuc-OligoGM1:
-
Fucosyl-GM1 oligosaccharide, IV2αFucII3Neu5Ac-Gg4, α-Fuc-(1-2)-β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-Glc
- Fuc-GM1:
-
Fucosyl-GM1, Fucosyl-GM1IV2αFucII3Neu5AcGg4Cer, α-Fuc-(1-2)-β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-β-Glc-(1-1)-Cer
- GM1:
-
II3Neu5Ac-Gg4Cer, β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-β-Glc-(1-1)-Cer
- GM2:
-
II3Neu5Ac-Gg3Cer, β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-β-Glc-(1-1)-Cer
- GM3:
-
II3Neu5Ac-Lac-Cer, α-Neu5Ac-(2-3)-β-Gal-(1-4)-β-Glc-(1-1)-Cer
- GD1a:
-
IV3Ne5AcII3Neu5Ac-Gg4Cer, α-Neu5Ac-(2-3)-β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-β-Glc-(1-1)-Cer
- HPTLC:
-
High-performance thin-layer chromatography
- MAPK:
-
Mitogen-activated protein kinase
- MS:
-
Mass spectrometry
- N2a:
-
Neuroblastoma Neuro2a cells
- NGF:
-
Nerve growth factor
- NMR:
-
Nuclear magnetic resonance
- OligoGM1:
-
GM1 oligosaccharide, II3Neu5Ac-Gg4, β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-Glc
- OligoGD1a:
-
GD1a oligosaccharide, IV3Ne5AcII3Neu5Ac-Gg4
- OligoGM3:
-
GM3 oligosaccharide, II3Neu5Ac-Lac, α-Neu5Ac-(2-3)-β-Gal-(1-4)-Glc
- OligoGM2:
-
GM2 oligosaccharide, II3Neu5Ac-Gg3, β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-Glc
- OligoGM1 w/o Glc:
-
GM1 oligosaccharide without glucose; β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-Gal
- P/S:
-
Penicillin/streptomycin
- pTrk:
-
Phosphorylated Trk
- PVDF:
-
Polyvinylidene difluoride
- reduced-OligoGM1:
-
GM1 oligosaccharide containing reduced glucose in position 1, β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-Glucitol
- TBS-T:
-
Tris-buffered saline containing 0.1% Tween-20
- Tyr:
-
Tyrosine
- Trk:
-
Neurotrophin tyrosin kinase receptor
- w/o:
-
Without
- WT:
-
Wild-type
References
Chester, M.A.: IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids–recommendations 1997. Eur. J. Biochem. 257, 293–298 (1998)
Svennerholm, L.: Composition of gangliosides from human brain. Nature 177, 524–525 (1956)
Sonnino, S., Brocca, P., Acquotti, D., Bernardi, A., Raimondi, L., Kiso, M., Ishida, H., Li, S.C., Li, Y.T.: The structural basis for the susceptibility of gangliosides to enzymatic degradation. Biosci. Rep. 19, 163–168 (1999)
Sonnino, S., Aureli, M., Grassi, S., Mauri, L., Prioni, S., Prinetti, A.: Lipid rafts in neurodegeneration and neuroprotection. Mol. Neurobiol. 50, 130–148 (2014)
Chiricozzi, E.: Plasma membrane glycosphingolipid signaling: a turning point. Glycoconj. J. 39, 99–105 (2022)
Sarmento, M.J., Ricardo, J.C., Amaro, M., Šachl, R.: Organization of gangliosides into membrane nanodomains. FEBS Lett. 594, 3668–3697 (2020)
Ledeen, R.W., Wu, G.: The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem. Sci. 40, 407–418 (2015)
Ledeen, R.W., Wu, G., Lu, Z.H., Kozireski-Chuback, D., Fang, Y.: The role of GM1 and other gangliosides in neuronal differentiation overview and new finding. Ann. N.Y. Acad. Sci. 845, 161–175 (1998)
Ledeen, R., Wu, G.: Gangliosides of the Nervous System. Methods Mol. Biol. 1804, 19–55 (2018)
Guo, Z.: Ganglioside GM1 and the Central Nervous System. Int. J. Mol. Sci. 24, (2023)
Chiricozzi, E., Lunghi, G., Di Biase, E., Fazzari, M., Sonnino, S., Mauri, L.: GM1 Ganglioside is a key factor in maintaining the mammalian neuronal functions avoiding neurodegeneration. Int. J. Mol. Sci. 21, (2020)
Brocca, P., Berthault, P., Sonnino, S.: Conformation of the oligosaccharide chain of G(M1) ganglioside in a carbohydrate-enriched surface. Biophys. J. 74, 309–318 (1998)
Brocca, P., Bernardi, A., Raimondi, L., Sonnino, S.: Modeling ganglioside headgroups by conformational analysis and molecular dynamics. Glycoconj. J. 17, 283–299 (2000)
Yagi-Utsumi, M., Kameda, T., Yamaguchi, Y., Kato, K.: NMR characterization of the interactions between lyso-GM1 aqueous micelles and amyloid beta. FEBS Lett. 584, 831–836 (2010)
Yagi-Utsumi, M., Kato, K.: Structural and dynamic views of GM1 ganglioside. Glycoconj. J. 32, 105–112 (2015)
Acquotti, D., Poppe, L., Dabrowski, J., Von der Lieth, C.W., Sonnino, S., Tettamanti, G.: Three-dimensional structure of the oligosaccharide chain of GM1 ganglioside revealed by a distance-mapping procedure: a rotating and laboratory frame nuclear overhauser enhancement investigation of native glycolipid in dimethyl sulfoxide and in water-dodecylphosphocholine solutions. J. Am. Chem. Soc. 7772–7778 (1990)
Chiricozzi, E., Di Biase, E., Lunghi, G., Fazzari, M., Loberto, N., Aureli, M., Mauri, L., Sonnino, S.: Turning the spotlight on the oligosaccharide chain of GM1 ganglioside. Glycoconj. J. 38, 101–117 (2021)
Chiricozzi, E., Pomè, D.Y., Maggioni, M., Di Biase, E., Parravicini, C., Palazzolo, L., Loberto, N., Eberini, I., Sonnino, S.: Role of the GM1 ganglioside oligosaccharide portion in the TrkA-dependent neurite sprouting in neuroblastoma cells. J. Neurochem. 143, 645–659 (2017)
Chiricozzi, E., Maggioni, M., di Biase, E., Lunghi, G., Fazzari, M., Loberto, N., Elisa, M., Scalvini, F.G., Tedeschi, G., Sonnino, S.: The Neuroprotective Role of the GM1 Oligosaccharide, II3Neu5Ac-Gg4, in neuroblastoma cells. Mol. Neurobiol. 56, 6673–6702 (2019)
Fazzari, M., Di Biase, E., Zaccagnini, L., Henriques, A., Callizot, N., Ciampa, M.G., Mauri, L., Carsana, E.V., Loberto, N., Aureli, M., Mari, L., Civera, M., Vasile, F., Sonnino, S., Bartels, T., Chiricozzi, E., Lunghi, G.: GM1 oligosaccharide efficacy against α-synuclein aggregation and toxicity in vitro. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1868, 159350 (2023)
Wiegandt, H., Bücking, H.W.: Carbohydrate components of extraneuronal gangliosides from bovine and human spleen, and bovine kidney. Eur. J. Biochem. 15, 287–292 (1970)
Tettamanti, G., Bonali, F., Marchesini, S., Zambotti, V.: A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim. Biophys. Acta 296, 160–170 (1973)
Acquotti, D., Cantù, L., Ragg, E., Sonnino, S.: Geometrical and conformational properties of ganglioside GalNAc-GD1a, IV4GalNAcIV3Neu5AcII3Neu5AcGgOse4Cer. Eur. J. Biochem. 225, 271–288 (1994)
Imamura, A., Yoshikawa, T., Komori, T., Ando, M., Ando, H., Wakao, M., Suda, Y., Ishida, H., Kiso, M.: Design and synthesis of versatile ganglioside probes for carbohydrate microarrays. Glycoconj. J. 25, 269–278 (2008)
Chiricozzi, E., Biase, E.D., Maggioni, M., Lunghi, G., Fazzari, M., Pomè, D.Y., Casellato, R., Loberto, N., Mauri, L., Sonnino, S.: GM1 promotes TrkA-mediated neuroblastoma cell differentiation by occupying a plasma membrane domain different from TrkA. J. Neurochem. 149, 231–241 (2019)
Sonnino, S., Cantù, L., Corti, M., Acquotti, D., Venerando, B.: Aggregative properties of gangliosides in solution. Chem. Phys. Lipids 71, 21–45 (1994)
Corti, M., Degiorgio, V., Ghidoni, R., Sonnino, S., Tettamanti, G.: Laser-light scattering investigation of the micellar properties of gangliosides. Chem. Phys. Lipids 26, 225–238 (1980)
Ulrich-Bott, B., Wiegandt, H.: Micellar properties of glycosphingolipids in aqueous media. J. Lipid Res. 25, 1233–1245 (1984)
Valsecchi, M., Chigorno, V., Sonnino, S., Tettamanti, G.: Rat cerebellar granule cells in culture associate and metabolize differently exogenous GM1 ganglioside molecular species containing a C18 or C20 long chain base. Chem. Phys. Lipids 60, 247–252 (1992)
Di Biase, E., Lunghi, G., Fazzari, M., Maggioni, M., Pomè, D.Y., Valsecchi, M., Samarani, M., Fato, P., Ciampa, M.G., Prioni, S., Mauri, L., Sonnino, S., Chiricozzi, E.: Gangliosides in the differentiation process of primary neurons: the specific role of GM1-oligosaccharide. Glycoconj. J. 37, 329–343 (2020)
Schengrund, C.L., Prouty, C.: Oligosaccharide portion of GM1 enhances process formation by S20Y neuroblastoma cells. J. Neurochem. 51, 277–282 (1988)
Schneider, C.A., Rasband, W.S., Eliceiri, K.W.: NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012)
Aureli, M., Bassi, R., Prinetti, A., Chiricozzi, E., Pappalardi, B., Chigorno, V., Di Muzio, N., Loberto, N., Sonnino, S.: Ionizing radiations increase the activity of the cell surface glycohydrolases and the plasma membrane ceramide content. Glycoconj. J. 29, 585–597 (2012)
Wehrman, T., He, X., Raab, B., Dukipatti, A., Blau, H., Garcia, K.C.: Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron 53, 25–38 (2007)
Park, S.J., Lee, J., Qi, Y., Kern, N.R., Lee, H.S., Jo, S., Joung, I., Joo, K., Im, W.: CHARMM-GUI Glycan Modeler for modeling and simulation of carbohydrates and glycoconjugates. Glycobiology 29, 320–331 (2019)
Naïm, M., Bhat, S., Rankin, K.N., Dennis, S., Chowdhury, S.F., Siddiqi, I., Drabik, P., Sulea, T., Bayly, C.I., Jakalian, A., Purisima, E.O.: Solvated interaction energy (SIE) for scoring protein-ligand binding affinities. 1. Exploring the parameter space. J. Chem. Inf. Model. 47, 122–133 (2007)
Biller, J.R., Elajaili, H., Meyer, V., Rosen, G.M., Eaton, S.S., Eaton, G.R.: Electron spin-lattice relaxation mechanisms of rapidly-tumbling nitroxide radicals. J. Magn. Reson. 236, 47–56 (2013)
Facci, L., Leon, A., Toffano, G., Sonnino, S., Ghidoni, R., Tettamanti, G.: Promotion of neuritogenesis in mouse neuroblastoma cells by exogenous gangliosides. Relationship between the effect and the cell association of ganglioside GM1. J. Neurochem. 42, 299–305 (1984)
Farooqui, T., Franklin, T., Pearl, D.K., Yates, A.J.: Ganglioside GM1 enhances induction by nerve growth factor of a putative dimer of TrkA. J. Neurochem. 68, 2348–2355 (1997)
Singleton, D.W., Lu, C.L., Colella, R., Roisen, F.J.: Promotion of neurite outgrowth by protein kinase inhibitors and ganglioside GM1 in neuroblastoma cells involves MAP kinase ERK1/2. Int. J. Dev. Neurosci. 18, 797–805 (2000)
Rabin, S.J., Bachis, A., Mocchetti, I.: Gangliosides activate Trk receptors by inducing the release of neurotrophins. J. Biol. Chem. 277, 49466–49472 (2002)
Duchemin, A.M., Ren, Q., Mo, L., Neff, N.H., Hadjiconstantinou, M.: GM1 ganglioside induces phosphorylation and activation of Trk and Erk in brain. J. Neurochem. 81, 696–707 (2002)
Da Silva, J.S., Hasegawa, T., Miyagi, T., Dotti, C.G., Abad-Rodriguez, J.: Asymmetric membrane ganglioside sialidase activity specifies axonal fate. Nat. Neurosci. 8, 606–615 (2005)
Mocchetti, I.: Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell. Mol. Life Sci. 62, 2283–2294 (2005)
Zakharova, I.O., Sokolova, T.V., Vlasova, Y.A., Furaev, V.V., Rychkova, M.P., Avrova, N.F.: GM1 ganglioside activates ERK1/2 and Akt downstream of Trk tyrosine kinase and protects PC12 cells against hydrogen peroxide toxicity. Neurochem. Res. 39, 2262–2275 (2014)
Huang, E.J., Reichardt, L.F.: Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 (2003)
Byrne, M.C., Ledeen, R.W., Roisen, F.J., Yorke, G., Sclafani, J.R.: Ganglioside-induced neuritogenesis: verification that gangliosides are the active agents, and comparison of molecular species. J. Neurochem. 41, 1214–1222 (1983)
Nagai, Y.: Functional roles of gangliosides in bio-signaling. Behav. Brain Res. 66, 99–104 (1995)
Skaper, S.D., Katoh-Semba, R., Varon, S.: GM1 ganglioside accelerates neurite outgrowth from primary peripheral and central neurons under selected culture conditions. Brain Res. 355, 19–26 (1985)
Valaperta, R., Valsecchi, M., Rocchetta, F., Aureli, M., Prioni, S., Prinetti, A., Chigorno, V., Sonnino, S.: Induction of axonal differentiation by silencing plasma membrane-associated sialidase Neu3 in neuroblastoma cells. J. Neurochem. 100, 708–719 (2007)
Pshezhetsky, A.V., Ashmarina, M.: Keeping it trim: roles of neuraminidases in CNS function. Glycoconj. J. 35, 375–386 (2018)
Ledeen, R.W., Wu, G., Cannella, M.S., Oderfeld-Nowak, B., Cuello, A.C.: Gangliosides as neurotrophic agents: studies on the mechanism of action. Acta Neurobiol. Exp. (Wars) 50, 439–449 (1990)
Ferrari, G., Anderson, B.L., Stephens, R.M., Kaplan, D.R., Greene, L.A.: Prevention of apoptotic neuronal death by GM1 ganglioside: Involvement of Trk neurotrophin receptors. J. Biol. Chem. 270, 3074–3080 (1995)
Mutoh, T., Tokuda, A., Miyadai, T., Hamaguchi, M., Fujiki, N.: Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. U.S.A. 92, 5087–5091 (1995)
Mutoh, T., Tokuda, A., Inokuchi, J., Kuriyama, M.: Glucosylceramide synthase inhibitor inhibits the action of nerve growth factor in PC12 cells. J. Biol. Chem. 273, 26001–26007 (1998)
Bachis, A., Rabin, S.J., Del Fiacco, M., Mocchetti, I.: Gangliosides prevent excitotoxicity through activation of TrkB receptor. Neurotox. Res. 4, 225–234 (2002)
Kappagantula, S., Andrews, M.R., Cheah, M., Abad-Rodriguez, J., Dotti, C.G., Fawcett, J.W.: Neu3 sialidase-mediated ganglioside conversion is necessary for axon regeneration and is blocked in CNS axons. J. Neurosci. 34, 2477–2492 (2014)
Manev, H., Favaron, M., Vicini, S., Guidotti, A., Costa, E.: Glutamate-induced neuronal death in primary cultures of cerebellar granule cells: protection by synthetic derivatives of endogenous sphingolipids. J. Pharmacol. Exp. Ther. 252, 419–427 (1990)
Costa, E., Armstrong, D., Guidotti, A., Kharlamov, A., Kiedrowski, L., Wroblewski, J.T.: Ganglioside GM1 and its semisynthetic lysogangliosides reduce glutamate neurotoxicity by a novel mechanism. Adv. Exp. Med. Biol. 341, 129–141 (1993)
Kharlamov, A., Guidotti, A., Costa, E., Hayes, R., Armstrong, D.: Semisynthetic sphingolipids prevent protein kinase C translocation and neuronal damage in the perifocal area following a photochemically induced thrombotic brain cortical lesion. J. Neurosci. 13, 2483–2494 (1993)
Saito, M., Berg, M.J., Guidotti, A., Marks, N.: Gangliosides attenuate ethanol-induced apoptosis in rat cerebellar granule neurons. Neurochem. Res. 24, 1107–1115 (1999)
Hadaczek, P., Wu, G., Sharma, N., Ciesielska, A., Bankiewicz, K., Davidow, A.L., Lu, Z.H., Forsayeth, J., Ledeen, R.W.: GDNF signaling implemented by GM1 ganglioside; failure in Parkinson’s disease and GM1-deficient murine model. Exp. Neurol. 263, 177–189 (2015)
Aureli, M., Mauri, L., Carsana, E.V., Dobi, D., Breviario, S., Lunghi, G., Sonnino, S.: Gangliosides and Cell Surface Ganglioside Metabolic Enzymes in the Nervous System. Adv. Neurobiol. 29, 305–332 (2023)
Cirillo, F., Ghiroldi, A., Fania, C., Piccoli, M., Torretta, E., Tettamanti, G., Gelfi, C., Anastasia, L.: NEU3 Sialidase Protein Interactors in the Plasma Membrane and in the Endosomes. J. Biol. Chem. 291, 10615–10624 (2016)
Venerando, B., Cestaro, B., Fiorilli, A., Ghidoni, R., Preti, A., Tettamanti, G.: Kinetics of Vibrio cholerae sialidase action on gangliosidic substrates at different supramolecular-organizational levels. Biochem. J. 203, 735–742 (1982)
Monti, E., Bassi, M.T., Papini, N., Riboni, M., Manzoni, M., Venerando, B., Croci, G., Preti, A., Ballabio, A., Tettamanti, G., Borsani, G.: Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem. J. 349, 343–351 (2000)
Hata, K., Wada, T., Hasegawa, A., Kiso, M., Miyagi, T.: Purification and characterization of a membrane-associated ganglioside sialidase from bovine brain. J. Biochem. 123, 899–905 (1998)
Hasegawa, T., Yamaguchi, K., Wada, T., Takeda, A., Itoyama, Y., Miyagi, T.: Molecular cloning of mouse ganglioside sialidase and its increased expression in Neuro2a cell differentiation. J. Biol. Chem. 275, 8007–8015 (2000)
Preti, A., Fiorilli, A., Lombardo, A., Caimi, L., Tettamanti, G.: Occurrence of sialyltransferase activity in the synaptosomal membranes prepared from calf brain cortex. J. Neurochem. 35, 281–296 (1980)
Crespo, P.M., Demichelis, V.T., Daniotti, J.L.: Neobiosynthesis of glycosphingolipids by plasma membrane-associated glycosyltransferases. J. Biol. Chem. 285, 29179–29190 (2010)
Ottico, E., Prinetti, A., Prioni, S., Giannotta, C., Basso, L., Chigorno, V., Sonnino, S.: Dynamics of membrane lipid domains in neuronal cells differentiated in culture. J. Lipid Res. 44, 2142–2151 (2003)
Varki, A., Cummings, R.D., Aebi, M., Packer, N.H., Seeberger, P.H., Esko, J.D., Stanley, P., Hart, G., Darvill, A., Kinoshita, T., Prestegard, J.J., Schnaar, R.L., Freeze, H.H., Marth, J.D., Bertozzi, C.R., Etzler, M.E., Frank, M., Vliegenthart, J.F., Lütteke, T., Perez, S., Bolton, E., Rudd, P., Paulson, J., Kanehisa, M., Toukach, P., Aoki-Kinoshita, K.F., Dell, A., Narimatsu, H., York, W., Taniguchi, N., Kornfeld, S.: Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 25, 1323–1324 (2015)
Funding
E.C. was supported by PSR 2019, University of Milano. L.M. was supported by RV_TAR16SSONN_M. I.E., L.P., Si.Sa. and O.B.M. were supported by MIUR “Progetto d’Eccellenza 2023–2025”. L.P. was supported by PSR 2022, University of Milano.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, investigation, analysis, and draft of the manuscript, M.F., G.L., E.D.B.; M.M., E.V.C., L.C., L.V., E.C., Si.Sa., O.B.M., L.P., I.E., M.A., N.L., M.G.C. Supervision, conceptualization, draft and revision of the manuscript, M.F., G.L., Sa.So., I.E., E.C.; GM1 and derivatives chemical synthesis, L.M., M.G.C., K.T., A.I., H.I., Docking Analysis: I.E., O.B.M., Si.Sa., L.P.; All authors have revised, read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interests
The authors declare that they have no conflicts of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fazzari, M., Lunghi, G., Di Biase, E. et al. GM1 structural requirements to mediate neuronal functions. Glycoconj J 40, 655–668 (2023). https://doi.org/10.1007/s10719-023-10141-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-023-10141-8