Abstract
Factors behind intraspecific variation in sensitivity to pathogens remain poorly understood. We investigated how geographical origin in two North European amphibians affects tolerance to infection by the chytrid fungus Batrachochytrium dendrobatidis (Bd), a generalist pathogen which has caused amphibian population declines worldwide. We exposed newly metamorphosed individuals of moor frog Rana arvalis and common toad Bufo bufo from two latitudinal regions to two different BdGPL strains. We measured survival and growth as infections may cause sub-lethal effects in fitness components even in the absence of mortality. Infection loads were higher in B. bufo than in R. arvalis, and smaller individuals had generally higher infection loads. B. bufo had high mortality in response to Bd infection, whereas there was little mortality in R. arvalis. Bd-mediated mortality was size-dependent and high-latitude individuals were smaller leading to high mortality in the northern B. bufo. Bd exposure led to sub-lethal effects in terms of reduced growth suggesting that individuals surviving the infection may have reduced fitness mediated by smaller body size. In both host species, the Swedish Bd strain caused stronger sublethal effects than the British strain. We suggest that high-latitude populations can be more vulnerable to chytrids than those from lower latitudes and discuss the possible mechanisms how body size and host geographical origin contribute to the present results.
Similar content being viewed by others
Introduction
Natural populations are increasingly affected by emerging infectious diseases (Daszak et al. 2000; Pennisi 2010; Fisher et al. 2012; Scheele et al. 2019). Many of the emerging diseases are caused by generalist and opportunistic fungal pathogens which can infect a wide range of host species (Wibbelt et al. 2010; Fisher et al. 2012; Lorch et al. 2016; More et al. 2018). The virulence of fungal pathogens often differs among host species, leading to population declines in some hosts while having no apparent effect on others (Casadevall 2007; Herczeg et al. 2021). There is evidence that populations of a host species may differ in their susceptibility, but apart from plant systems few studies have addressed this question in detail (Ebert 2008; Laine et al. 2011; Bradley et al. 2015; Martin-Torrijos et al. 2017).
The chytrid fungus Batrachochytrium dendrobatidis (Bd), causing the disease chytridiomycosis in amphibians, is a generalist pathogen which has caused the decline of over 500 amphibian species, including the presumed extinction of 90 species (Berger et al. 1998; Skerratt et al. 2007; Lips 2016; Scheele et al. 2019). Bd is endemic to East Asia, and severe outbreaks of chytridiomycosis have been observed in the Americas and Australia (Lips 2016; O’Hanlon et al. 2018; Scheele et al. 2019). There is considerable variation in virulence among genetic strains of Bd (Farrer et al. 2011; Bataille et al. 2013; Greenspan et al. 2018) and BdGPL, the global panzootic lineage originating in Eastern Asia, has caused most of the chytridiomycosis outbreaks (O’Hanlon et al. 2018). While genetic variation within BdGPL is relatively limited (O’Hanlon et al. 2018), there is evidence for virulence differences also between BdGPL strains (Becker et al. 2017; Burrow et al. 2017; Dang et al 2017, Greener 2020).
As a generalist pathogen, Bd infects a wide range of amphibian species (Lips 2016; Scheele et al. 2019). Not all species develop chytridiomycosis; many are resistant to the disease and can clear the infection, while others can tolerate high infection loads without developing the disease (Fisher et al. 2009a; Gahl et al. 2012; Ellison et al. 2014; Scheele et al. 2017). Similarly, geographical populations of the same species can differ in their susceptibility to Bd (Savage and Zamudio 2011; Bradley et al. 2015; Kosch et al. 2019). These differences can be due to genetic differences in traits like immune response and behavior (Richards-Zawacki 2010), and are in some cases linked with direct Bd-mediated selection (Savage and Zamudio 2016; Savage et al. 2018). Although infection does not cause direct mortality in the resistant and tolerant populations and species, sub-lethal fitness effects such as decreased growth have been detected (Bielby et al. 2015; Burrow et al. 2017).
Climate-related latitudinal divergence is an important structuring force of intraspecific genetic variation (e.g., Hewitt 2000, Conover et al. 2009), but its potential role in mediating host–pathogen interactions has received little attention. Two lines of evidence suggest that amphibian populations living at high latitudes in the northern hemisphere may be especially vulnerable to disease. Firstly, due to post-glacial colonization patterns northern populations often harbor less genetic variation (Hewitt 2000). In many amphibians, this is true also for immunogenetic variation in major histocompatibility (MHC) genes (Zeisset and Beebee 2014; Cortázar-Chinarro et al. 2017, 2022; Höglund et al. 2022), which is associated with Bd resistance (Savage and Zamudio 2011; Savage et al. 2018; Kosch et al. 2019). Furthermore, pathogen species richness and abundance are significant predictors of adaptive MHC variation (Wang et al. 2017). As pathogen richness and abundance decrease towards colder climates (Schemske et al. 2009), populations at higher latitudes may encounter lower diversity and a lower number of pathogens which may lead to increased drift and loss of adaptive immunogenetic variation in these populations (Cortázar-Chinarro et al. 2017). Secondly, time-constrained high-latitude environments select for high larval development rates (Palo et al. 2003; Luquet et al. 2019), which in amphibians can trade-off with disease resistance (Johnson et al. 2011; Woodhams et al. 2016) and immune response (Gervasi and Foufopoulos 2007; Murillo-Rincon et al. 2017). While all these factors may contribute to lower ability to withstand novel pathogens in high-latitude populations, no studies on disease resistance between latitudinal populations have been made.
Bd is widely spread in southern Scandinavia (e.g., Meurling et al. 2020), but very few experimental studies focusing on sensitivity of high-latitude amphibians to Bd infection have been made (Cortazar-Chinarro et al. 2022). Here we conducted a laboratory common garden experiment to examine inter- and intraspecific population differences in response to Bd infection in Scandinavian amphibians. Our aims were three-fold. First, we investigated the responses of two common north European amphibians (moor frog Rana arvalis and common toad Bufo bufo) to Bd infection. Second, we investigated if the responses differ between southern and northern Scandinavian populations of these species. We predicted that due to lower genetic variation and higher development rates in the north, the northern populations are more sensitive to Bd infection. Finally, we evaluated if the amphibian responses to infection differ between two geographically separated Bd lineages. To this end, we infected newly metamorphosed amphibians and measured their survival and growth during a 30-day period.
Methods
Animal rearing
Both R. arvalis (hereafter Ra) and B. bufo (hereafter Bb) are widespread amphibians in Europe occurring up to the polar circle in the north (Sillero et al. 2014). Both species are explosive breeders and mate in early spring. In southern Sweden, Bd prevalence in breeding adults is 15.3% (n = 288) and 3.4% (n = 941) in Ra and Bb, respectively (Meurling et al. 2020).
Eggs of both species were collected in April 2016 at two sites in Skåne county in southernmost Sweden and May 2016 at two sites in in Norrbotten county in northern Sweden (Fig. 1; Table S1). We collected ca. ten eggs from each of ten different clutches at each site. All collection ponds in the south were screened for Bd in 2015 and found negative (over 100 breeding adults tested; Meurling et al. 2020). In the north we tested in total four ponds in 2016 (two of which were included in the present study) and did not find Bd in any of them (Meurling et al. 2020).
The eggs and tadpoles were reared until metamorphosis in walk-in climate-controlled rooms at Uppsala University in plastic tanks filled with \(20\) l reconstituted soft water (RSW; NaHCO3, CaSO4, MgSO4 and KCl added to deionized water; APHA 1985). Each clutch was kept in a separate tank under 18:6 h light/dark regime at 19 °C. The tadpoles were fed ad libitum spinach and fish flakes and water was changed every third day. At metamorphic climax (stage 42; Gosner 1960), the animals were moved to another tank of the same size with access to aquatic and terrestrial (aquarium sand) habitat and a shelter. Four days after completion of tail absorption (stage 46), the animals were transported to the sealed experimental facilities at the Swedish Institute for Veterinary Science, Uppsala, where they were kept individually in 1.2 l plastic tanks lined with moist paper towels and a lid of a plastic bottle as a shelter. The metamorphs were kept in these tanks until the end of the experiment and fed fruit flies and crickets ad libitum under 18:6 h light/dark regime at 19 °C. The condition of each animal was checked daily and the tanks were cleaned every third day.
Infection experiment
The infection treatments were conducted after one week of acclimatization at the experimental facility. The experimental animals were exposed to one of two isolates of Bd-GPL (UK or SWE) or a sham infection consisting of culture medium (Table 1). The UK isolate (UKMal 01) was isolated from a wild alpine newt (Icthyosaura alpestris) in the UK in 2008. The Swedish isolate (SWED-40-5) originated from a wild green toad (Bufotes viridis) in Malmö municipality in southern Sweden in 2015. The animals were exposed individually for 5 h to 200 µl culture media containing a dosage of 60 000 zoospores in \(30\) ml of RSW. The control group (C) was exposed for 5 h to an equivalent volume of RSW and culture media without Bd spores. Altogether, we treated 74 (25 in SWE, 24 in UK and 25 in C treatment) southern and 46 (16 SWE, 14 UK, 16 C) northern Ra. The corresponding numbers for Bb were 64 (21, 19, 24) southern and 90 (31, 31, 28) northern individuals.
After exposure the animals were monitored for 30 days. Animals showing irreversible signs of chytridiomycosis (loss of righting function) were euthanized with an overdose of MS222. Wet body mass was measured immediately before exposure and at the end of the experiment (or at death) with a microbalance to the precision of 0.1 mg. At the end of the experiment, the surviving animals were euthanized and stored in 96% ethanol at 4 °C.
DNA extraction and qPCR analyses
To confirm infection status, we assessed the presence of Bd by using qPCR. DNA was extracted from a hind leg using a Prepman Ultra method described in Boyle et al. (2004). Presence of Bd was assessed by amplifying the internal transcribed spacer (ITS)-5.8S rRNA region (Boyle et al 2004). 25 µl reactions containing 12.5 µl 2 × Taqman Master Mix (Applied Biosystem, ref. 4,318,157), 2.25 µl 10 µM each of forward and reverse primers, 0.625 µl 10 µM MGB probe and 5µl of DNA (diluted × 10 in water) were run. Each sample was run in triplicate. An exogenous internal positive control (IPC; Hyatt et al. 2007) was added to one well in each triplicate (1 µl 10XExo IPC master mix and 0.5 µl 50XExo IPC DNA to each sample; VICTIM dye, Applied Biosystems ref. 4,304,662) to avoid false negatives due to inhibitors. The qPCR assays were run on a Biorad CFX96 Real Time System machine using amplification conditions described in Boyle et al. (2004) with standards of 0.1, 1, 10 and 100 genomic equivalents (GE). An individual was recorded as positive if at least one of the triplicate samples exhibited a positive signal (i.e. an exponential amplification curve). If the IPC showed signs of inhibition (i.e. was negative), the sample was rerun once. If the IPC was still negative the sample was assigned as failed and removed from the data set (Table 1). The above-mentioned standards were used to create a standard curve which was then used to calculate the infection intensity for each individual expressed in genome equivalents (GE).
For the statistical analysis of the infection, we used the log10 (zero values were replaced by 0.001, one tenth of the lowest measured non-zero value) of GE as a measure of infection load (IL). Molecular analyses of IL in 16 Ra and 23 Bb individuals failed (Table 1). Among the successful analyses, we found no presence of Bd for one Ra and one Bb, both of which were infected by the UK strain. All of these individuals were excluded from the statistical analyses involving IL.
Statistical analyses
All analyses were conducted in R 3.5.2 (R Core Team 2018). Survival was analysed using Generalised Linear Models with a binomial error distribution and a logit link function. IL and growth were analysed using linear models. The assumptions for the linear models were checked using the model diagnostic plots in R, and these plots were also used to identify outliers. In one of the analyses, one outlier was removed (Table S1). In Bb, the growth data were log-transformed due to heteroscedasticity. Daily growth was defined as (mass at day 30—mass at exposure)/30 in both species. In order to be able to study the non-lethal effects of Bd infection, dead individuals (3 Ra and 55 Bb) were removed from the growth analyses.
The models that best explained differences in IL, survival and growth were selected using 170 bidirectional eliminations (stepAIC function in R package MASS) starting from the full model: Response ~ Region + Bd-infection + Size at infection + IL + Interactions, where region and infection were treated as factors and size and IL as covariates. The models were first run investigating the effect of Bd-infection by grouping the two Bd treatments together and comparing them with the control treatment (factor levels: infected and controls), and second by comparing the effects of the two Bd-strains (UK and SWE) excluding the controls from the model. If any interactions were included in the selected model, but none of them was close to significance (p < 0.1), the model selection was rerun without interactions to avoid unnecessarily complicated model. Type III sums of squares were used when interactions were included (Anova function in R package car). Covariates were standardized with Student’s method before the analyses.
Results
The qPCR analyses showed high infection success: only two of the successfully analysed individuals from the exposed groups were negative to Bd infection at the end of the experiment (one Bb and one Ra; Table 1) and were removed from the analyses. Thirteen control individuals were also found Bd positive (Table 1). However, IL in these individuals was always very low (mean 0.08 ± 0.014 (SE) genomic equivalents, range 0.03–0.235), and we find it likely that these samples were contaminated during sample processing at the end of the experiment. For the statistical analysis, IL in these individuals was therefore considered to be 0.
At the time of Bd infection, northern animals were significantly smaller than southern animals both in Ra (F1, 118 = 26.35, p < 0.001) and in Bb (F1, 152 = 159.14 p < 0.001; Fig. 2). There were no size differences between the infection treatments at the time of infection (Ra: F2, 117 = 0.09, p = 0.91, Bb: F2, 151 = 0.09, p = 0.916).
Infection load
For Ra, the selected model was IL ~ Size + Region + Size × Region (Table S1a). We found a significant interaction between size at infection and region (F1, 69 = 6.5, p = 0.013), size having a negative effect on IL in the northern region, but no effect in the southern region (Fig. 3a).
Infection load (log10 average GE, at the end of treatment) as the function of size at infection with SWE or UK Bd strain in a R. arvalis (\(n=73\)) and b B. bufo (\(n=91\)). Lines give the predictions of the model. Filled dots and solid lines represent the northern region (N), while open dots and dashed lines represent the southern region (S)
Bb had higher IL than Ra (F1, 163 = 15.09, p < 0.001; Fig S1). The selected model for Bb was IL ~ Bd-strain + Size + Bd-strain × Size (Table S1b). Size had a significant negative effect on infection load (F1, 87 = 64.45, p < 0.001, Fig. 3b). The interaction between Bd-strain and size was close to significant (F1, 87 = 3.66, p = 0.059), large toadlets infected with SWE strain having somewhat higher loads than large individuals infected with UK strain.
Survival
Survival in southern Ra was complete, whereas three infected individuals from the northern region died during the experiment, resulting in 92.9% survival in UK and 87.5% in SWE treatment. As survival was complete in the control treatment and in the southern region these were excluded from the statistical analyses. The selected model for the infected individuals in the northern region was Survival ~ Size (Table S3a) indicating poorer survival of smaller individuals in the two infection treatments in the northern region (χ2 1, 26 = 6.49 p = 0.011; Fig. 4a). However, this result should be interpreted with caution as it is based on only three cases of mortality.
Survival as a function of size at infection with SWE or UK Bd strain for a R. arvalis from the northern region (\(n=28\)). b Survival in B. bufo as function of infection load and size at infection, where dark and pale dots represent the northern and southern regions, respectively (\(n=91\)). Curve (a) and surfaces (b) give the predictions of the model. Blue dots and surface (in b) refer to SWE strain and red dots and surface to UK strain. In b some pale dots are hidden among the dark dots in the lower corner in the front (high infection load and small size)
While all Bb in the control treatment survived the experiment, there was considerable mortality in the infection treatments. Furthermore, survival was higher in the southern (66.7% in SWE and 89.5% in UK) than in the northern region (38.7% in SWE and 12.9% in UK). Due to 100% survival in the control treatment we excluded it from the full model. We also excluded the interaction term between region and size, as well as between region and IL, as the size and IL range in the northern region covered only a small subset of the range in the southern region, and including these interactions may have led to problematic extrapolations. Survival of Bb in the two infection treatments was best explained by the model Survival ~ Bd-strain + Size + IL + Bd-strain × IL (Table S3b). Initial size had a strong positive effect on survival (F1, 86 = 13.10, p < 0.001, Fig. 4b), whereas IL had a strong negative effect (F1, 86 = 27.61, p < 0.001). In addition, the significant interaction between Bd strain and infection load (F1, 86 = 7.05, p = 0.009) was due to SWE strain causing higher mortality at high IL than UK strain (Fig. 4b).
Growth
The model explaining growth best for Ra was Growth ~ IL + Size (Table S4a) showing that larger individuals had higher growth rates (F1,109 = 7.76, p = 0.006; Fig. 5a), and individuals with higher infection load suffered a more severe growth decline (F1,109 = 46.6, p < 0.001; Fig. 5b). An additional analysis showed that this was due to infected individuals (combining the two Bd-strains and removing IL from the model) having lower growth than those in the control treatment (F1,113 = 38.1, p < 0.001). The comparison between the two Bd-strains revealed that individuals infected with SWE strain have lower growth than those infected with UK strain (F1,67 = 6.95, p = 0.01; Fig. 5b, Table S4b) and that a higher infection load results in a more severe growth decline (F1,67 = 5.10, p = 0.027; Fig. 5b, Table S4b).
Growth (g/day) of R. arvalis as a function of a size at infection in different infection treatments, and b infection load (log10 average GE), at the end of experiment. Filled and open dots, and solid and dashed lines, represent the northern and southern regions, respectively. The blue and red lines give the predicted growth of individuals infected with SWE and UK strains, respectively (model in Table S4b, \(n=71\)), evaluated for average infection load in a and average size in b. The black and green line gives the predicted growth, evaluated for average infection load of the infected and control treatments, respectively, in a, while the black line is evaluated average size of all individuals in b (model in Table S4a, \(n=112\))
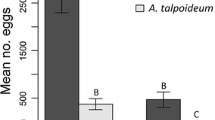
The model best explaining growth in Bb was Growth ~ Bd-infection + Region + Size + Bd-infection × Region + Bd-infection × Size (Table S4c). Larger Bb had higher growth rate (F1,92 = 56.15, p < 0.001; Fig. 6). Bd-infection reduced growth (F1,92 = 9.03, p = 0.003; Fig. 6) and the positive size effect was weaker in Bd-infected individuals (Infection × Size: F1,92 = 8.22, p = 0.005; Fig. 6). When analysed separately as a covariate, IL explained the growth decline independent of individual size and region (F1,85 = 5.65, p = 0.020, data not shown). The absence of interactions between infection load and both size and region is explained by IL indirectly considering both size and region, as IL was lower in larger individuals, and as northern individuals were smaller.
Growth (g/day) of B. bufo as a function of size at infection with SWE or UK Bd strain or in the control treatment. Filled and open dots, and solid and dashed lines, represent the northern and southern regions, respectively. The black and green lines give the predicted growth of infected and control treatments, respectively (model in Table S4c, \(n=98\)). The blue and red lines give the predicted growth of individuals infected with SWE and UK strains, respectively (model in Table S4d, \(n=46\))
Including IL as a covariate in the model comparing Bb growth in the two infection treatments resulted in a large, possibly overfit model with four significant interactions but sample size of only 37. We therefore run the model selection without IL (Table S4d). We found that individuals infected with SWE were more negatively affected than those infected with UK and this effect was stronger in large individuals (Strain × Size: F1,41 = 6.15, p = 0.017; Fig. 6). Northern individuals had lower growth than those from the south (F1,41 = 9.87, p = 0.003; Fig. 6).
Discussion
We found that Bd infection lowered survival especially in Bb and in the northern region. Our analyses suggest that the survival differences between the regions were largely mediated by body size, smaller individuals being more sensitive to Bd. While the results were qualitatively similar for Ra, these results should be interpreted with caution due to considerably lower mortality in this species. Furthermore, we found that Bd infection led to sub-lethal effects in terms of reduced growth, suggesting that individuals surviving the infection may have lower fitness mediated by their smaller body size. These results indicate that Bd infection may have both direct and indirect effects on amphibian populations and that high- latitude populations may run a higher risk of negative effects than their low-latitude counterparts.
Both species became infected in our experiment, but IL in Ra was lower and Bd-mediated mortality was only a fraction of the mortality experienced by Bb. However, Ra individuals did not clear the infection during the 30 day observation period. These results agree with previous studies showing that brown frogs have higher tolerance to Bd, while bufonids are more susceptible to Bd-infection (Bosch and Martínez-Solano 2006; Garner et al. 2009; Gahl et al. 2012; Balaz et al. 2014; Bielby et al. 2015). When comparing susceptibility to infection and Bd-mediated mortality between two anuran species, Bielby et al. (2015) found that R. temporaria, a sister species to Ra, was resistant to infection even at high doses, while almost all Bb became infected and showed high dose-dependent mortality. Since Ra has higher infection prevalence in the wild (Meurling et al. 2020) and higher infection tolerance (this study), we suggest that Ra may act as a reservoir species and a possible vector for Bd-transmission to more sensitive species such as Bb. Indeed, Kärvemo et al. (2019) showed that Bb populations coexisting with Ra had higher Bd-prevalence than populations breeding in ponds without Ra.
We found a clear difference in survival between northern and southern populations especially in Bb. Northern individuals were smaller at the time of infection than southern individuals, and our analyses suggest that the survival difference was mainly mediated by body size. As we raised the tadpoles under common garden conditions, the differences in body mass most likely have a genetic origin. The higher vulnerability of smaller individuals to Bd agrees with previous studies (Garner et al. 2009; Bradley et al. 2015; Burrow et al. 2017). Smaller individuals may have less resources and less developed immune system to fight the infection increasing their vulnerability to disease (Møller et al. 1998; Lochmiller and Deerenberg 2000; Burrow et al. 2017). This is supported by the fact that smaller individuals had higher ILs. Smaller individuals may also be more vulnerable to Bd-mediated water loss as they have larger surface area to body mass ratio. Increased water loss via sloughing is an important symptom in chytridiomycosis, which may render smaller individuals more sensitive to Bd infection (Russo et al. 2018; Wu et al. 2019).
In addition to the size differences, two further, not mutually exclusive, explanations may further explain higher mortality in the northern populations. Firstly, northern populations may have less effective immune systems because of reduced genetic variation due to postglacial colonization processes (Hewitt 2000, see Cortazar-Chinarro et al. 2017, Rödin-Mörch et al. 2019 for Ra, Thörn et al. 2021 for Bb) or lower pathogen abundance at higher latitudes (Schemske et al. 2009). This hypothesis gains support from the fact that MHC variation in both our study species is lower at higher latitudes (Cortázar-Chinarro et al. 2017, 2022). Moreover, Bd-mediated survival in Bb seems to be linked with certain MHC alleles (Cortázar-Chinarro et al. 2022), as also found in other species (Savage and Zamudio 2011; Savage et al. 2018; Kosch et al. 2019). Secondly, higher larval development rates in the northern populations may trade off with disease resistance (Johnson et al. 2011; Woodhams et al. 2016). Also this hypothesis is indirectly supported by the facts that more time-constrained populations have higher development rates in both our study species (Luquet et al. 2015, 2019) and that Ra tadpoles experimentally induced to develop faster have weaker immune response (Murillo-Rincon et al. 2017). Additional studies focusing on Bd resistance in known MHC and developmental genotypes would be highly interesting.
Bd infection had clear negative effects on growth in both species. As body size is positively related to fitness in juvenile amphibians (Earl and Whiteman 2015), these results suggest that Bd may have sublethal fitness effects. For example, hibernation success is often positively related to body size and failing to reach a sufficient size before hibernation can greatly reduce overwinter survival (Altwegg and Reyer 2003). This can be especially detrimental at higher latitudes where the hibernation period can reach eight months. Small body size may also lead to higher risk of predation, delayed maturation and lower ability to compete for resources and mates (reviewed in Earl and Whiteman 2015). In the long run, these effects may decrease population growth rate and ability to cope with environmental changes such as higher temperature due to climate change. In our case, even if survival of Ra was not strongly affected by Bd infection, the sublethal effects of infection mediated by body size may still lower individual and population fitness.
We found relatively little evidence for differences in pathogenicity between the two Bd isolates. Nevertheless, we found significant strain × size interaction in survival of Bb with larger toadlets being more vulnerable to infection with SWE strain than with UK strain. These results suggest that individuals infected with UK strain may relatively quickly reach a size where the lethality of Bd is reduced, while Bd-mediated mortality induced by SWE strain is less size-dependent. This effect can be especially clear in the southern individuals which are larger at metamorphosis, while the smaller northern individuals stay longer in the vulnerable size classes. Importantly, in both host species SWE strain had a stronger negative effect on growth, suggesting that SWE infected individuals may spend a longer time in the vulnerable size class, and that potential sublethal fitness effects mediated by body-size differences are more severe in SWE infection. The reasons for the virulence differences between the BdGPL strains are unclear, but earlier studies have emphasized the importance of zoospore production and sporangia size as well as differences in genes related to colonization ability and cell invasiveness (Fisher et al. 2009b; Greener et al. 2020). Moreover, the host species from which the Bd strain was isolated can play a role (Belasen et al. 2022). Interestingly, in a recent meta-analyses Belasen et al. (2022) found that the geographical proximity of the BdGPL source locality and the host population played very little role in Bd-induced mortality.
A potential caveat in our study is that we used laboratory-raised (but wild-collected) individuals. Lab-raised individuals may not have as diverse skin microbiota as individuals living in the wild which are exposed to more diverse microbial community. Indeed, captive amphibians often have a reduced and less varied bacterial community than wild populations of the same species (Antwis et al. 2014; Bataille et al. 2016). As skin microbiome plays an important role in defending against fungal and other pathogens, this could impact the ability of amphibians reared in captivity to respond to Bd infection (Harris et al. 2009; Walke et al. 2015; Madison et al. 2017; Woodhams et al. 2018). We currently lack knowledge on the skin microbiomes of our study species and if these differ between geographical regions. Microbiome studies are needed for additional insight on factors behind the high mortality found in this study.
Bd is widespread in the southern parts of Sweden (Kärvemo et al. 2018, 2019; Meurling et al. 2020). However, in a pattern similar to much of Europe (Lips 2016; Scheele et al. 2019), no cases of chytridiomycosis or unusual die-offs have been found in Sweden. Our experimental results suggest that even though no negative effects of the infection have been seen in the wild, this might not be the complete picture. It is currently unclear how well the present results translate to natural conditions, but we note that Bd causes sublethal effects in terms of reduced movements and body condition in wild Scandinavian amphibians (Kärvemo et al. 2019, 2020). Furthermore, the lethality of Bd is highly dependent on environmental conditions, including temperature (e.g., Novakowski et al. 2016, Mosher et al. 2018, Cohen et al. 2019), and relatively minor elevations in mortality may risk long-term survival of Bd-infected amphibian populations (Muths et al. 2011; Spitzen-van der Sluijs et al. 2017). Two important conclusions can be drawn. Firstly, very few Bd surveys have been conducted in northernmost Scandinavia, and the results so far have been negative (Meurling et al. 2020). As populations at higher latitudes can be more vulnerable to infection, it is important to investigate the occurrence of Bd in these areas and, if still possible, prevent or limit the possible northward spread of the fungus. Secondly, we showed that infection leads to higher mortality and reduced body size. These, in turn, can lead to reduced population growth rates in the long-term even in the absence of major mortality effects. As the potential negative effects of Bd on population growth can be relatively subtle and difficult to detect (Doddington et al. 2013, Spitzen-van der Sluijs et al. 2017, Mosher et al. 2018), long-term monitoring of amphibian populations is of high importance.
Data availability
The data will be deposited in DRYAD upon acceptance.
References
Altwegg R, Reyer HU (2003) Patterns of natural selection on size at metamorphosis in water frogs. Evolution 57:872–882
Antwis RE, Haworth RL, Engelmoer DJ et al (2014) Ex situ diet influences the bacterial community associated with the skin of red-eyed tree frogs (Agalychnis callidryas). PLoS ONE 9:e85563
APHA (1985) Standard methods for the examination of water and wastewater, 16th edn. American Public Health Association, Washington, DC
Balaz V, Voros J, Civis P et al (2014) Assessing risk and guidance on monitoring of Batrachochytrium dendrobatidis in Europe through identification of taxonomic selectivity of infection. Conserv Biol 28:213–223
Bataille A, Fong JJ, Cha M et al (2013) Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Mol Ecol 22:4196–4209
Bataille A, Lee-Cruz L, Tripathi B et al (2016) Microbiome variation across amphibian skin regions: Implications for chytridiomycosis mitigation efforts. Microb Ecol 71:221–232
Becker CG, Greenspan Se, Tracy KE (2017) Variation in phenotype and virulence among enzootic and panzootic amphibian chytrid lineages. Fungal Ecol 26:45–50
Becker CG, Bletz MC, Greenspan SE et al (2019) Low-load pathogen spillover predicts shifts in skin microbiome and survival of a terrestrial-breeding amphibian. Proc R Soc B 286:20191114
Belasen AM, Rusell ID, Zamudio KR, Bletz MC (2022) Endemic lineages of Batrachochytrium dendrobatidis are associated with reduced chytridiomycosis-induced mortality in amphibians: evidence from a meta-analysis of experimental infection studies. Front Vet Sci 9:756686
Berger L, Speare R, Daszak P et al (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95:9031–9036
Bielby J, Fisher MC, Clare FC et al (2015) Host species vary in infection probability, sub-lethal effects, and costs of immune response when exposed to an amphibian parasite. Sci Rep 5:10828
Bosch J, Martínez-Solano I (2006) Chytrid fungus infection related to unusual mortalities of Salamandra salamandra and Bufo bufo in the Peñalara Natural Park, Spain. Oryx 40:84–89
Boyle DG, Boyle DB, Olsen V et al (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org 60:141–148
Bradley PW, Gervasi SS, Hua J et al (2015) Differences in sensitivity to the fungal pathogen Batrachochytrium dendrobatidis among amphibian populations. Conserv Biol 29:134–1356
Burrow AK, Rumschlag SL, Boone MD (2017) Host size influences the effects of four isolates of an amphibian chytrid fungus. Ecol Evol 7:9196–9202
Casadevall A (2007) Determinants of virulence in the pathogenic fungi. Fungal Biol Rev 21:130–132
Cohen JM, McMahon TA, Ramsay C et al (2019) Impacts of thermal mismatches on chytrid fungus Batrachochytrium dendrobatidis prevalence are moderated by life stage, body size, elevation and latitude. Ecol Lett 22:817–825
Cortázar-Chinarro M, Lattenkamp EZ, Meyer-Lucht Y et al (2017) Drift, selection, or migration? Processes affecting genetic differentiation and variation along a latitudinal gradient in an amphibian. BMC Evol Biol 17:189
Cortazar-Chinarro M, Meurling S, Schroens L et al (2022) MHC variation and haplotype associated survival in response to experimental infection of two Bd-GPL strains along a latitudinal gradient. Front Ecol Evol 10:915271
Dang TD, Searle CL, Blaustein AR (2017) Virulence variation among strains of the emerging infectious fungus Batrachochytrium dendrobatidis in multiple amphibian host species. Dis Aquat Org 124:233–239
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife– Threats to biodiversity and human health. Science 287:443–449
Doddington BJ, Bosch J, Oliver JA et al (2013) Context-dependent amphibian host population response to an invading pathogen. Ecology 94:1795–1804
Earl JE, Whiteman HH (2015) Are commonly used fitness predictors aAccurate? A meta-analysis of amphibian size and age at metamorphosis. Copeia 103:297–309
Ebert D (2008) Host-parasite coevolution. insights from the Daphnia-parasite model system. Curr Opin Microbiol 11:290–301
Ellison AR, Tunstall T, DiRenzo GV et al (2014) More than skin deep: functional genomic basis for resistance to amphibian chytridiomycosis. Genome Biol Evol 7:286–298
Farrer RA, Weinert LA, Bielby J et al (2011) Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc Natl Acad Sci USA 108:18732–18736
Fisher MC, Bosch J, Yin Z et al (2009a) Proteomic and phenotypic profiling of the amphibian pathogen Batrachochytrium dendrobatidis shows that genotype is linked to virulence. Mol Ecol 18:415–429
Fisher MC, Garner TW, Walker SF (2009b) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol 63:291–310
Fisher MC, Henk DA, Briggs CJ et al (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186
Gahl MK, Longcore JE, Houlahan JE (2012) Varying responses of rortheastern North American amphibians to the chytrid pathogen Batrachochytrium dendrobatidis. Conserv Biol 26:135–141
Garner TWJ, Walker S, Bosch J et al (2009) Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos 118:783–791
Gervasi S, Foufopoulos J (2007) Costs of plasticity: Responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct Ecol 22:100–108
Gosner KL (1960) A Simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Greener MS, Verbrugghe E, Kelly M et al (2020) Presence of low virulence chytrid fungi could protect European amphibians from more deadly strains. Nat Comm 11:5393
Greenspan SE, Lambertini C, Carvalo T et al (2018) Hybrids of amphian chytrid show high virulence in native hosts. Sci Rep 8:9600
Harris RN, Lauer A, Simon MA, Banning JL, Alford RA (2009) Addition of antifungal skin bacteria to salamanders ameliorates the effects of chytridiomycosis. Dis Aquat Org 83:11–16
Herczeg D, Ujszegi J, Kasler A et al (2021) Host-multiparasite interactions in amphibians: a review. Parasit Vectors 14:296
Hewitt G (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913
Höglund J, Bolender L, Cortazar-Chinarro M, Meurling S, Laurila A, Hermaniuk A, Dufresnes C (2022) Low neutral and immunogenetic diversity in northern fringe populations of the green toad Bufotes viridis: implications for conservation. Conserv Genet 23:139–149
Hyatt AD, Boyle DG, Olsen V et al (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Org 73:175–192
Johnson PTJ, Kellermanns E, Bowerman J (2011) Critical windows of disease risk: amphibian pathology driven by developmental changes in host resistance and tolerance. Funct Ecol 25:726–734
Kärvemo S, Meurling S, Berger D et al (2018) Effects of host species and environmental factors on the prevalence of Batrachochytrium dendrobatidis in northern Europe. PLoS ONE 13:e0199852
Kärvemo S, Laurila A, Höglund J (2019) Urban environment and reservoir host species are associated with Batrachochytrium dendrobatidis infection prevalence in the common toad. Dis Aquat Org 134:33–42
Kärvemo S, Wikström G, Widenfalk LA et al (2020) Chytrid fungus dynamics and infections associated with movement distances in a red-listed amphibian. J Zool 311:164–174
Kosch TA, Silva CNS, Brannelly LA et al (2019) Genetic potential for disease resistance in critically endangered amphibians decimated by chytridiomycosis. Anim Conserv 22:238–250
Laine A-L, Burdon JJ, Dodds PN et al (2011) Spatial variation in disease resistance: from molecuales to metapopulations. J Ecol 99:96–112
Lips KR (2016) Overview of chytrid emergence and impacts on amphibians. Phil Trans R Soc B 371:20150465
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos 88:87–98
Lorch JM, Knowles S, Lankton JS et al (2016) Snake fungal disease: an emerging threat to wild snakes. Phil Trans R Soc B 371:20150457
Luquet E, Léna JP, Miaud C et al (2015) Phenotypic divergence of the common toad (Bufo bufo) along an altitudinal gradient: evidence for local adaptation. Heredity 114:69–79
Luquet E, Rödin Mörch P, Cortázar-Chinarro M et al (2019) Post-glacial colonization routes coincide with a life-history breakpoint along a latitudinal gradient. J Evol Biol 32:356–368
Madison JD, Berg EA, Abarca JG et al (2017) Characterization of Batrachochytrium dendrobatidis inhibiting bacteria from amphibian populations in Costa Rica. Front Microbiol 8:290
Martin-Torrijos L, Campos Llach M, Pou Rovira Q et al (2017) Resistance to crayfish plague, Aphanomyces astaci (Oomycota) in the endangered freshwater crayfish species Austropotamobius pallipes. PLoS ONE 12:e0181226
Meurling S, Kärvemo S, Chondrelli N et al (2020) Occurrence of Batrachochytrium dendrobatidis in Sweden: higher infection prevalence in southern species. Dis Aquat Org 140:209–218
Miller AP, Christe P, Erritzoe J et al (1998) Condition, disease and immune defence. Oikos 83:301–306
More S, Angel Miranda M, Bicout D et al (2018) Risk of survival, establishment and spread of Batrachochytrium salamandrivorans (Bsal) in the EU. EFSA J 16:e05259
Mosher BA, Bailey LL, Muths E et al (2018) Host-pathogen metapopualtion suggest high elevation refugia for boreal toads. Ecol Appl 28:926–937
Murillo-Rincon AP, Laurila A, Orizaola G (2017) Compensating for delayed hatching reduces offspring immune response and increases life-history costs. Oikos 126:565–571
Muths E, Scherer RD, Pilliod DS (2011) Compensatroy effects of recruitment and survival when amphibians are perturbed by disease. Ecol Appl 48:873–879
Nowakowski AJ, Whitfield SM, Eskew EA et al (2016) Infection risk decreases with increasing mismatch in host and pathogen environmental tolerances. Ecol Lett 19:1051–1061
O’Hanlon SJ, Rieux A, Farrer RA et al (2018) Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360:621–627
Palo JU, O’Hara RB, Laugen AT et al (2003) Latitudinal divergence of common frog (Rana temporaria) life history traits by natural selection: evidence from a comparison of molecular and quantitative genetic data. Mol Ecol 12:1963–1978
Pennisi E (2010) Armed and dangerous. Science 327:804–805
R Core Team (2018) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Richards-Zawacki CL (2010) Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc R Soc B 277:519–528
Rödin-Mörch P, Luquet E, Meyer-Lucht Y et al (2019) Latitudinal divergence in a widespread amphibian: Contrasting patterns of neutral and adpative genomic variation. Mol Ecol 28:2996–3011
Russo CJM, Ohmer MEB, Cramp RL, Franklin CE (2018) A pathogenic skin fungus and sloughing exacerbate cutaneous water loss in amphibians. J Exp Biol 221:167445
Savage AE, Zamudio KR (2011) MHC genotypes associate with resistance to a frog-killing fungus. Proc Natl Acad Sci USA 108:16705–16710
Savage AE, Zamudio KR (2016) Adaptive tolerance to a pathogenic fungus drives major histocompatibility complex evolution in natural amphibian populations. Proc R Soc B 283:20153115
Savage AE, Mulder KP, Torret T et al (2018) Lost but not forgotten: MHC genotypes predict overwinter survival despite depauparate MHC diversity in a declining frog. Conserv Genet 19:309–322
Scheele BC, Hunter DA, Brannelly LA et al (2017) Reservoir-host amplification of disease impact in an endangered amphibian. Conserv Biol 31:592–600
Scheele BC, Pasmans F, Skerratt LF et al (2019) Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363:1459–1463
Schemske DW, Mittelbach GG, Cornell HV et al (2009) Is there a latitudinal gradient in the importance of biotic interactions? Annu Rev Ecol Evol Syst 40:245–269
Sillero N, Campos J, Bonardi A et al (2014) Updated distribution and biogeography of amphibians and reptiles of Europe. Amph-Reptil 35:1–31
Skerratt LF, Berger L, Speare R et al (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134
Spitzen van der Sluijs A, Canessa S, Martel A (2017) Fragile coexistence of a global chytrid pathogen with amphibian populations is mediated by environment and demography. Proc R Soc B 284:20171444
Thörn F, Rödin-Mörch P, Cortazar-Chinarro M et al (2021) The effects of drift and selection on latitudinal genetic variation in Scandinavian common toads (Bufo bufo) following postglacial recolonization. Heredity 126:656–667
Voyles J, Woodhams DC, Saenz V et al (2018) Shifts in disease dynamics in a tropical amphibian assemblage are not due to pathogen attenuation. Science 359:1517
Walke JB, Becker MH, Loftus SC et al (2015) Community structure and function of amphibian skin microbes: an experiment with bullfrogs exposed to a chytrid fungus. PLoS ONE 10:e0139848
Wang SP, Liu CH, Wilson AB et al (2017) Pathogen richness and abundance predict patterns of adaptive major histocompatibility complex variation in insular amphibians. Mol Ecol 26:4671–4685
Wibbelt G, Kurth A, Hellmann D et al (2010) White-nose syndrome fungus (Geomyces destructans) in bats, Europe. Emerg Infect Dis 16:1237–1243
Woodhams DC, Bletz M, Kueneman J et al (2016) Managing amphibian disease with skin microbiota. Trends Microbiol 24:161–164
Woodhams DC, LaBumbard BC, Barnhart KL et al (2018) Prodigiosin, violacein, and volatile organic compounds produced by widespread cutaneous bacteria of amphibians can inhibit two Batrachochytrium fungal pathogens. Microb Ecol 75:1049–1062
Wu NC, McKercher C, Cramp RL et al (2019) Mechanistic basis for loss of water balance in green tree frogs infected with a fungal pathogen. Am J Physiol Regul Integr Comp Physiol 317:R301–R311
Zeisset I, Beebee TJC (2014) Drift rather than selection dominates MHC Class II Allelic diversity patterns at the biogeographical range scale in natterjack toads Bufo calamita. PLoS ONE 9:e100176
Acknowledgements
We thank Lola Brooks and Trent Garner for discussions and providing the Bd-strains, and David Lesbarreres and an anonymous reviewer for constructive comments on the manuscript.
Funding
Open access funding provided by Uppsala University. Funding from the Swedish Research Council Formas (215-2014-294), Swedish Research Council (621-2013-4503), Stiftelsen Oscar och Lili Lamms Minne and Stiftelsen för zoologisk forskning is acknowledged.
Author information
Authors and Affiliations
Contributions
SM, MCC, JH and AL conceived and designed the experiments, SM, MCC and DÅ performed the experiments and EÅ provided advice and logistic help, SM, PRM and MS analysed the data, SM and AL wrote the paper with input from all the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. The study was conducted with a permit (C28/15) from Uppsala ethical committee for animal experiments and collection permits from the county administrative boards in Skåne and Norrbotten.
Additional information
Communicated by Bryan Brown.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meurling, S., Siljestam, M., Cortazar-Chinarro, M. et al. Body size mediates latitudinal population differences in the response to chytrid fungus infection in two amphibians. Oecologia 204, 71–81 (2024). https://doi.org/10.1007/s00442-023-05489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-023-05489-5