Abstract
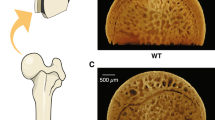
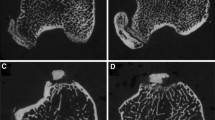
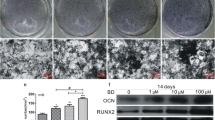
Vascular dysfunction contributes to the development of osteopenia in hypertensive patients, as decreased blood supply to bones results in tissue damage and dysfunction. The effect of anti-hypertensive medicines on bone mass in hypertensive individuals is inconclusive because of the varied mechanism of their action, and suggests that reducing blood pressure (BP) alone is insufficient to enhance bone mass in hypertension. Pentoxifylline (PTX), a hemorheological drug, improves blood flow by reducing blood viscosity and angiogenesis, also has an osteogenic effect. We hypothesized that improving vascular function is critical to increasing bone mass in hypertension. To test this, we screened various anti-hypertensive drugs for their in vitro osteogenic effect, from which timolol and hydralazine were selected. In adult female spontaneously hypertensive rats (SHRs), timolol and hydralazine did not improve vascular function and bone mass, but PTX improved both. In female SHR animals, PTX restored bone mass, strength and mineralization, up to the level of normotensive control rats. In addition, we observed lower blood vasculature in the femur of adult SHR animals, and PTX restored them. PTX also restored the bone vascular and angiogenesis parameters that had been impaired in OVX SHR compared to sham SHR. This study demonstrates the importance of vascular function in addition to increased bone mass for improving bone health as achieved by PTX without affecting BP, and suggests a promising treatment option for osteoporosis in hypertensive patients, particularly at-risk postmenopausal women.








Similar content being viewed by others
References
Pérez-Castrillón JL, Martín-Escudero JC, Alvarez Manzanares P et al (2005) Hypertension as a risk factor for hip fracture [2]. Am J Hypertens 18:146–147
Ye Z, Lu H, Liu P (2017) Association between essential hypertension and bone mineral density: a systematic review and meta-analysis. Oncotarget 8:68916–68927. https://doi.org/10.18632/oncotarget.20325
Zhang R, Yin H, Yang M et al (2023) Advanced progress of the relationship between antihypertensive drugs and bone metabolism. Hypertension 80(11):2255–2264. https://doi.org/10.1161/hypertensionaha.123.21648
Afghani A, Johnson CA (2006) Resting blood pressure and bone mineral content are inversely related in overweight and obese hispanic women. Am J Hypertens 19:286–292. https://doi.org/10.1016/j.amjhyper.2005.10.024
Canoy D, Harvey NC, Prieto-Alhambra D et al (2022) Elevated blood pressure, antihypertensive medications and bone health in the population: revisiting old hypotheses and exploring future research directions. Osteoporos Int 33:315–326. https://doi.org/10.1007/s00198-021-06190-0
Lerman LO, Kurtz TW, Touyz RM et al (2019) Animal models of hypertension: a scientific statement from the American Heart Association. Hypertension 73:e87–e120. https://doi.org/10.1161/HYP.0000000000000090
Louis WJ, Howes LG (1990) Genealogy of the spontaneously hypertensive rat and wistar-kyoto rat strains: implications for studies of inherited hypertension. J Cardiovasc Pharmacol 16:S1–S5. https://doi.org/10.1097/00005344-199006167-00002
Hawlitschek C, Brendel J, Gabriel P et al (2022) Antihypertensive and cardioprotective effects of different monotherapies and combination therapies in young spontaneously hypertensive rats—A pilot study: antihypertensive and cardioprotective effects of different monotherapies and combination therapies. Saudi J Biol Sci 29:339–345. https://doi.org/10.1016/j.sjbs.2021.08.093
Bastos MF, Brilhante FV, Bezerra JP et al (2010) Trabecular bone area and bone healing in spontaneously hypertensive rats. A histometric study. Braz Oral Res 24:170–176. https://doi.org/10.1590/S1806-83242010000200008
Kang KY, Kang Y, Kim M et al (2013) The effects of antihypertensive Drugs on bone mineral density in ovariectomized mice. J Korean Med Sci 28:1139–1144. https://doi.org/10.3346/jkms.2013.28.8.1139
Sato T, Arai M, Goto S, Togari A (2010) Effects of propranolol on bone metabolism in spontaneously hypertensive rats. J Pharmacol Exp Ther 334:99–105. https://doi.org/10.1124/jpet.110.167643
Shimizu H, Nakagami H, Yasumasa N et al (2012) Cilnidipine, but not amlodipine, ameliorates osteoporosis in ovariectomized hypertensive rats through inhibition of the N-type calcium channel. Hypertens Res 35:77–81. https://doi.org/10.1038/hr.2011.143
Castoldi G, Carletti R, Ippolito S et al (2022) Angiotensin II modulates calcium/phosphate excretion in experimental model of hypertension: focus on bone. Biomedicines 10(11):2928. https://doi.org/10.3390/biomedicines10112928
Zhang YF, Wang YXJ, Griffith JF et al (2009) Proximal femur bone marrow blood perfusion indices are reduced in hypertensive rats: a dynamic contrast-enhanced MRI study. J Magn Reson Imaging 30:1139–1144. https://doi.org/10.1002/jmri.21954
Ha JS, Cho HM, Lee HJ, Kim S, Do (2019) Bilateral avascular necrosis of the femoral head in a patient with asymptomatic adrenal incidentaloma. Hip Pelvis 31:120–123. https://doi.org/10.5371/hp.2019.31.2.120
Hong N, Du XK (2004) Avascular necrosis of bone in severe acute respiratory syndrome. Clin Radiol 59:602–608. https://doi.org/10.1016/j.crad.2003.12.008
Tsai H-L, Chang J-W, Lu J-H, Liu C-S (2022) Epidemiology and risk factors associated with avascular necrosis in patients with autoimmune Diseases: a nationwide study. Korean J Intern Med 37:864–876. https://doi.org/10.3904/kjim.2020.098
Benigni A, Cassis P, Remuzzi G (2010) Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2:247–257
Sparks MA, Crowley SD, Gurley SB et al (2014) Classical renin-angiotensin system in kidney physiology. Compr Physiol 4:1201–1228. https://doi.org/10.1002/cphy.c130040
Ames MK, Atkins CE, Pitt B (2019) The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med 33:363–382
McCarty MF, O’Keefe JH, DiNicolantonio JJ (2016) Pentoxifylline for vascular health: a brief review of the literature. Open Hear 3:e000365. https://doi.org/10.1136/openhrt-2015-000365
Ohshima N, Sato M (1981) Effect of pentoxifylline on microvascular blood flow velocity. Angiology 32:752–763. https://doi.org/10.1177/000331978103201103
Pal S, Porwal K, Singh H et al (2019) Reversal of osteopenia in ovariectomized rats by pentoxifylline: evidence of osteogenic and osteo-angiogenic roles of the drug. Calcif Tissue Int 105:294–307. https://doi.org/10.1007/s00223-019-00567-4
Zaydun G, Tomiyama H, Hashimoto H et al (2006) Menopause is an Independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis 184:137–142. https://doi.org/10.1016/j.atherosclerosis.2005.03.043
Mercuro G, Zoncu S, Saiu F et al (2004) Menopause induced by oophorectomy reveals a role of ovarian estrogen on the maintenance of pressure homeostasis. Maturitas 47:131–138. https://doi.org/10.1016/S0378-5122(03)00252-4
Sherwood A, Park SB, Hughes JW et al (2010) Cardiovascular hemodynamics during stress in premenopausal versus postmenopausal women. Menopause 17:403–409. https://doi.org/10.1097/gme.0b013e3181b9b061
Trivedi S, Srivastava K, Gupta A et al (2020) A quantitative method to determine osteogenic differentiation aptness of scaffold. J Oral Biol Craniofacial Res 10:158–160. https://doi.org/10.1016/j.jobcr.2020.04.006
Pal S, Rashid M, Singh SK et al (2020) Skeletal restoration by phosphodiesterase 5 inhibitors in osteopenic mice: evidence of osteoanabolic and osteoangiogenic effects of the drugs. Bone 135:115305. https://doi.org/10.1016/j.bone.2020.115305
Sharma S, Porwal K, Kulkarni C et al (2022) Diosmin, a citrus fruit-derived phlebotonic bioflavonoid protects rats from chronic kidney disease-induced loss of bone mass and strength without deteriorating the renal function. Food Funct 13:2184–2199. https://doi.org/10.1039/d1fo03867b
Porwal K, Pal S, Tewari D et al (2019) Increased bone marrow-specific adipogenesis by clofazimine causes impaired fracture healing, osteopenia, and osteonecrosis without extraskeletal effects in rats. Toxicol Sci 172:167–180. https://doi.org/10.1093/toxsci/kfz172
Lima R, Wofford M, Reckelhoff JF (2012) Hypertension in postmenopausal women. Curr Hypertens Rep 14:254–260. https://doi.org/10.1007/s11906-012-0260-0
Ji M, Yu Q (2015) Primary osteoporosis in postmenopausal women. Chronic Dis Transl Med 1:9–13. https://doi.org/10.1016/j.cdtm.2015.02.006
Noirrit-Esclassan E, Valera M-C, Tremollieres F et al (2021) Critical role of estrogens on bone homeostasis in both male and female: from physiology to medical implications. Int J Mol Sci 22:1568. https://doi.org/10.3390/ijms22041568
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23:576–581
Manolagas SC, O’Brien CA, Almeida M (2013) The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 9:699–712
Wang J, Gao Y, Cheng P et al (2017) CD31hiEmcnhi vessels support new trabecular bone formation at the frontier growth area in the bone defect repair process. Sci Rep 7(1):4990. https://doi.org/10.1038/s41598-017-04150-5
Han S, Oh M, Yoon S et al (2017) Risk stratification for avascular necrosis of the femoral head after internal fixation of femoral neck fractures by post-operative bone SPECT/CT. Nucl Med Mol Imaging 51:49–57. https://doi.org/10.1007/s13139-016-0443-8
Steffen RT, Athanasou NA, Gill HS, Murray DW (2010) Avascular necrosis associated with fracture of the femoral neck after hip resurfacing: histological assessment of femoral bone from retrieval specimens. J Bone Jt Surg - Ser B 92:787–793. https://doi.org/10.1302/0301-620X.92B6.23377
Matthews AH, Stitson D (2020) Osteonecrosis (Avascular Necrosis). StatPearls Publishing, Florida
Lee TC, Burghardt AJ, Yao W et al (2014) Improved trabecular bone structure of 20-month-old male spontaneously hypertensive rats. Calcif Tissue Int 95:282–291. https://doi.org/10.1007/s00223-014-9893-0
Pinto YM, Paul M, Ganten D (1998) Lessons from rat models of hypertension: from goldblatt to genetic engineering. Cardiovasc Res 39:77–88
Lowenthal DT, Hobbs D, Affrime MB et al (1980) Prazosin kinetics and effectiveness in renal failure. Clin Pharmacol Ther 27:779–783. https://doi.org/10.1038/clpt.1980.110
Fourtillan JB, Courtois P, Lefebvre MA, Girault J (1981) Pharmacokinetics of oral timolol studied by mass fragmentography. Eur J Clin Pharmacol 19:193–196. https://doi.org/10.1007/BF00561948
Jee WS, Yao W (2001) Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 1:193–207
Liu XL, Li CL, Lu WW et al (2015) Skeletal site-specific response to ovariectomy in a rat model: change in bone density and microarchitecture. Clin Oral Implants Res 26:392–398. https://doi.org/10.1111/clr.12360
Yousefzadeh N, Kashfi K, Jeddi S, Ghasemi A (2020) Ovariectomized rat model of osteoporosis: a practical guide. EXCLI J 19:89–107
Tiyasatkulkovit W, Promruk W, Rojviriya C et al (2019) Impairment of bone microstructure and upregulation of osteoclastogenic markers in spontaneously hypertensive rats. Sci Rep 9(1):12293. https://doi.org/10.1038/s41598-019-48797-8
Ribatti D, D’Amati A (2023) Bone angiocrine factors. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2023.1244372. 11:
Oinonen L, Tikkakoski A, Koskela J et al (2021) Parathyroid hormone may play a role in the pathophysiology of primary hypertension. Endocr Connect 10:54–65. https://doi.org/10.1530/EC-20-0446
Ammann P, Rizzoli R (2003) Bone strength and its determinants. Osteoporos Int 14:13–18
Ammann P, Rizzoli R, Meyer JM, Bonjour JP (1996) Bone density and shape as determinants of bone strength in IGF-I and/or pamidronate-treated ovariectomized rats. Osteoporos Int 6:219–227. https://doi.org/10.1007/BF01622738
Hart NH, Nimphius S, Rantalainen T et al (2017) Mechanical basis of bone strength: influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact 17:114–139
Acknowledgements
Authors acknowledge the erudite suggestions of Prof. Asit R. Mridha, AIIMS, New Delhi for conducting histological assessments of bone samples.
Funding
Council of Scientific and Industrial Research (MLP 2035), Government of India to Naibedya Chattopadhyay. CDRI communication number for this article is 10703.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Subhashis Pal, Shivani Sharma, Konica Porwal, Mahesh C. Tiwari, Yasir A. Khan, Saroj Kumar, Navin Kumar, Naibedya Chattopadhyay declare that they have no conflict of Interest.
Human and Animal Rights and Informed Consent statements
All animal experiments were performed following the ethical standards of The International Union for Conservation of Nature (IUCN) and approved by institutional committee for control and supervision of experiments on animals [The Institutional Animal Ethics Committee (IAEC) approval number for this study is CDRI/IAEC/2017/312)]. . The IAEC has been constituted by the Committee for the Control and Supervision of Experiments on Animals (CCSEA), the Government of India in which the Socially Aware Nominee of the CCSEA monitors all aspects of animal welfare and rights.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pal, S., Sharma, S., Porwal, K. et al. The Role of Osteogenic Effect and Vascular Function in Bone Health in Hypertensive Rats: A Study of Anti-hypertensive and Hemorheologic Drugs. Calcif Tissue Int 114, 295–309 (2024). https://doi.org/10.1007/s00223-023-01170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01170-4