Abstract
We present the results of our various attempts to prepare the purported room-temperature and ambient-pressure superconducting compound 'Pb9Cu(PO4)6O' (LK-99). We experimented with various starting materials and used several synthesis techniques, such as reactions in sealed silica tubes or sintering in air, to prepare the reported phase. Repetition of the exact synthesis procedure reported by Lee et al failed to reproduce the superconducting phase yielding only a multiphase sample. None of our prepared samples exhibit Meissner effect or levitation. Very importantly, only a small amount of copper was detected in the samples of the phase designated 'LK-99'. Dark gray flakes were found in some samples that reacted to a permanent magnet at room temperature, reminiscing of 'half-levitation.' Magnetic measurement reveal that all samples are diamagnetic in the temperature range of 2–325 K. At 2 K, a weak soft ferromagnetic behavior is observed, the origin of which is unknown.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
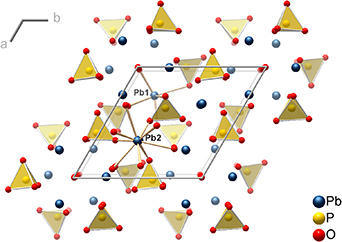
A very recent claim of (above) room-temperature superconductivity (RTSC) at ambient pressure by a group of Korean researchers on 22 July 2023, sparked a new wave of interest in investigating ambient pressure RTSC [1, 2]. The group claimed to have achieved this in 'LK-99', a copper-substituted lead oxyapatite with the composition Pb10−x Cux (PO4)6O (0.9 < x < 1.1). It is a derivative of the known insulating parent compound Pb10(PO4)6O [3], also known as oxypyromorphite (figure 1), and was reported by Lee et al to crystallize in the lead-apatite family of structures with a hexagonal lattice (space group P63/m, no. 176, figure 1) [1, 2]. Although no structure determination was carried out, copper(II) cations were supposed to occupy some of the nine-coordinated lead(II) cation positions (Pb2).
Figure 1. Crystal structure of Pb10(PO4)6O viewed along the crystallographic c-axis. The atoms Pb1 and Pb2 are coordinated by seven and nine oxygen atoms, respectively. Only one oxygen atom per formula unit is not part of phosphate tetrahedra and statistically distributed over several sites along the c-axis with 25% occupancy.
Download figure:
Standard image High-resolution imageThe sample by Lee et al [1, 2] was prepared by a unique metathesis reaction starting from an equimolar mixture of Pb2OSO4 (mineral Lanarkite) and Cu3P in a sealed silica tube at high temperature to yield Pb9Cu(PO4)6O. However, since off-stoichiometric (with respect to the target phase) amounts of reactants were used, a lot of side phases particularly Cu2S also resulted along with the desired phase. The scientific community immediately set out to verify this claim and made several attempts to replicate the observation of RTSC [4–16]. However, all experimental reports have tended to confirm that 'LK-99' is not superconducting. Until now, there is no experimental procedure that would yield a homogenous single-phase and stoichiometrically pure Pb9Cu(PO4)6O. Moreover, Wu et al [16] observed very interesting transitions at ∼380 K in both resistivity as well as in magnetic susceptibility for pure Cu2S that matches to those found in the sample 'LK-99', which contained significant Cu2S portions. Thus, it is important to produce a pure sample of Pb9Cu(PO4)6O, especially free from Cu2S impurities, to probe the intrinsic properties of the compound.
We performed detailed experiments to reproduce the compound via the originally reported route, as well as using several new alternative approaches that should theoretically yield no by-products. However, all of our modified synthetic routes failed to yield the superconducting 'LK-99'phase. Using detailed compositional analysis, we highlight the reluctance of copper to be incorporated into the lead oxyapatite. This work also outlines the difficulties in preparing a homogeneous single-phase material, which otherwise appears simple in theory. In addition, we characterized the sample by magnetic measurements and made the interesting observation of 'half-levitation' of some particles over a permanent magnet at room temperature.
2. Experimental
2.1. Synthesis
Syntheses targeting Pb10−x Cux (PO4)6O were carried out using several routes listed below. The experiments I–V were performed in evacuated silica tubes at 925 °C with dwell times of 10–20 h. The experiments VI and VII were performed in air using platinum crucibles at the same temperature. The starting materials Pb2OSO4 and Cu3P were prepared by solid state reactions exactly as reported by Lee et al [1, 2] Additionally, high purity Cu3P used as P source in experiment I(b) was prepared using an efficient ionothermal method [17]. CuO (abcr, 99.99%), Cu powder (Sigma Aldrich, 99.9%, reduced in H2 stream at 400 °C before use), P2O5 (Riedel-de Haën, 98%), PbO (abcr, 99.999%) and (NH4)2HPO4 (Thermoscientific, 98%) were used after an initial check for phase purity by powder x-ray diffraction (PXRD). Monoclinic Pb3(PO4)2 and triclinic Cu3(PO4)2 were prepared by direct reaction of stoichiometric mixtures of (NH4)2HPO4 with PbO and CuO, respectively. The mixtures were heated in platinum crucibles in air at 900 °C for a period of 20 h. The synthesized compounds were checked for their phase purity by PXRD before using them as starting materials for further synthesis. The following seven synthesis routes were tested (ST = sealed tube):
- I.2 Pb2OSO4 + 2 Cu3P → 1/3 Pb9Cu(PO4)6O + 2 Cu2S + 5/3 CuO + Pb (ST)
- II.8 PbSO4 + PbO + 3Cu + 6 Pred → Pb9Cu(PO4)6O + 1 Cu2S + SO2 ↑ + 3 O2 ↑ (ST)
- III.6 Pb2OSO4 + CuO + 6 Pred → Pb9Cu(PO4)6O + 3 PbS + 3 O2 ↑ (ST)
- IV.9 PbO + CuO + 3 P2O5 → Pb9Cu(PO4)6O (in air)
- V.3 Pb3(PO4)2 + CuO → Pb9Cu(PO4)6O (in air)
- VI.8 Pb3(PO4)2 +Cu3(PO4)2 + 3 PbO → 3 Pb9Cu(PO4)6O (in air)
- VII.9 PbO + CuO + 6 NH4H2PO4 → Pb9Cu(PO4)6O + 6 NH3 ↑ + 9 H2 ↑ (in air)
2.2. Chemical analysis
Semi-quantitative elemental analysis of the samples was performed with energy-dispersive x-ray (EDX) spectroscopy using a scanning electron microscope (SEM; SU8020, Hitachi) equipped with a silicon drift detector (X-MaxN, Oxford).
2.3. X-ray diffraction
PXRD data (CuKα1, λ = 154.059 pm, T = 296(1) K) were collected using an X'Pert Pro diffractometer (PANalytical, Bragg–Brentano geometry, curved Ge-(111) monochromator, fixed divergence slits, PIXcel detector). The samples for the PXRD measurements were ground and fixed on a single-crystal silicon sample holder.
2.4. Magnetic measurements
Magnetization data was collected using a vibrating sample magnetometer on a cryogenic-free measurement system (CFMS, Cryogenics Ltd.). Variable temperature magnetization was collected in the temperature range 2–325 K in an applied magnetic field of 50 Oe in zero field cooled (zfc) and field cooled (fc) conditions. Data was collected in warming runs. Isothermal magnetization at 2 and 325 K was recorded in an applied field of ±1 T.
3. Results and discussion
3.1. Synthesis and phase analysis
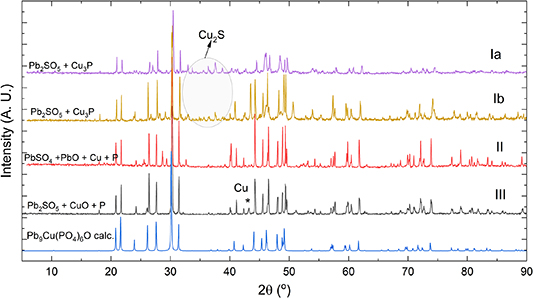
Details of all the synthesis routes adapted for preparation of LK-99 phase and their subsequent results are tabulated in table 1. The PXRD patterns of all the solid products are shown in figures 2–4. These experimental patterns of the Pb10(PO4)6O type phase match very well with the theoretical pattern of Pb9Cu(PO4)6O as well as with the pattern of the 'LK-99' phase reported by Lee et al [1, 2] There is a slight shift in the diffraction peaks of the measured patterns towards smaller d-values, which could be misinterpreted as a hint on the substitution of a large lead atom by a smaller copper atom. However, this shift is large at low diffraction angles and vanishing towards higher diffraction angles, typical for absorption effects.
Figure 2. PXRD patterns (CuKα1) of the solid products obtained from syntheses I–III (black, red, yellow and purple lines) in comparison with the calculated powder pattern of Pb9Cu(PO4)6O (bottom most, blue line), indicating that such a phase is the main product.
Download figure:
Standard image High-resolution imageFigure 3. PXRD patterns (CuKα1) of products obtained from synthetic route IV (black) and V (red) in comparison to the calculated powder pattern of Pb3(PO4)2 (blue).
Download figure:
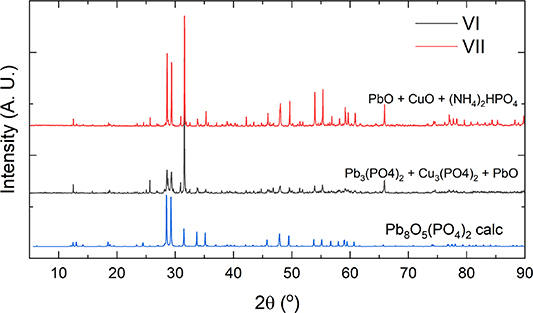
Standard image High-resolution imageFigure 4. PXRD patterns of products obtained from synthetic route VI (black) and VII (red) in comparison to the calculated powder pattern of Pb8O5(PO4)2 (blue).
Download figure:
Standard image High-resolution imageTable 1. Details of the synthesis routes adapted for the preparation of 'LK-99'. Cu3P was prepared by solid state reaction (*) or by ionothermal synthesis (#).
| Expt. | Starting materials | PXRD of the solid product | SEM-EDX | Remarks |
|---|---|---|---|---|
| Ia | Pb2(SO4)O, Cu3P* | Pb10(PO4)6O type phase + Cu2S | Only traces of copper detected in the Pb10(PO4)6O crystals | No levitation |
| Ib | Pb2(SO4)O, Cu3P# | Pb10(PO4)6O type phase + Cu2S | Small amount of copper detected in the Pb10(PO4)6O crystals | No levitation |
| II | PbSO4, PbO, Cu, Pred | Pb10(PO4)6O type phase + small impurity | No copper detected in the Pb10(PO4)6O crystals | No levitation |
| III | Pb2(SO4)O, CuO, Pred | Pb10(PO4)6O type phase (nearly pure), traces of Cu; PbS crystals at the cold end of the tube | No or only trace copper detected in the Pb10(PO4)6O crystals; some PbS and Cu2S | No levitation |
| IV | PbO, CuO, P2O5 | Monoclinic Pb3(PO4)2 | Pb:P = 3:2, no copper detected in the crystals | No levitation |
| V | Pb3(PO4)2, CuO | Monoclinic Pb3(PO4)2 | Not performed | Light grayish powder |
| VI | Pb3(PO4)2, Cu3(PO4)2, PbO | Pb9(PO4)6 and Pb3Cu3(PO4)4 | Pb:P = 1.6 and a copper-rich phase | transparent greenish/yellow crystals |
| VII | PbO, CuO, (NH4)2HPO4 | Mostly Pb8O5(PO4)2 | No copper detected in the crystals; Pb:P ≈ 4 | Transparent yellowish crystals |
In our experiments, a Pb10(PO4)6O type phase only formed when lead sulfates (PbSO4 or Pb2OSO4) were used as a precursor (I–III), otherwise different compounds resulted, most commonly monoclinic Pb3(PO4)2 [18] or Pb8(PO4)2 [3]. However, the use of sulfates is not mandatory in general, as Krivovichev and Burns were able to synthesize Pb10(PO4)6O from a mixture of PbO and NH4H2PO4 by heating to 950 °C in platinum crucibles and then slowly cooling to 50 °C over 150 h [3]. However, the crystals of Pb10(PO4)6O only occurred as a minor phase.
We shall now only focus on the products that yielded the Pb10(PO4)6O type phases (experiments I–III). Although the PXRD patterns appears promising, visual examination of the products under optical microscope clearly shows the multiphase nature of all samples. Along with dark greyish-looking lumps, there are regions, which are pale-yellowish transparent or opaque rust-colored (figure 5), depending on the reactants used. An inhomogeneous and multiphase product is naturally expected for sample I, which was made following the published protocol to obtain 'LK-99' (see reaction equation above). Cu2S (or CuS) must appear as a co-product in much higher amount than the target phase, which is clearly observed in the PXRD pattern shown by Lee et al. Other authors, Kumar et al [4] and Guo et al [19], also encountered this co-product. If only the dark grey appearing part of the sample is chosen, a clean looking PXRD pattern of Pb10−x Cux (PO4)6O is obtained. This might also have been done by Kumar et al [20] and Hou et al [15], yet they did not explicitly describe the sample preparation for PXRD measurements.
Figure 5. Optical images of the products obtained during different synthesis on mm grid. All the samples contain dark chunks, rust-colored and yellowish mass along with transparent pale-yellowish crystals.
Download figure:
Standard image High-resolution imageOur reaction III, using Pb2OSO4, CuO and Pred, resulted in essentially pure Pb10(PO4)6O, decorated with some small copper globules. At the cold end of the tube, PbS crystals were deposited, which do not contaminate the target phase. The visible formation of elemental copper already indicated that no or only little of this element has been included into the lead oxyapatite.
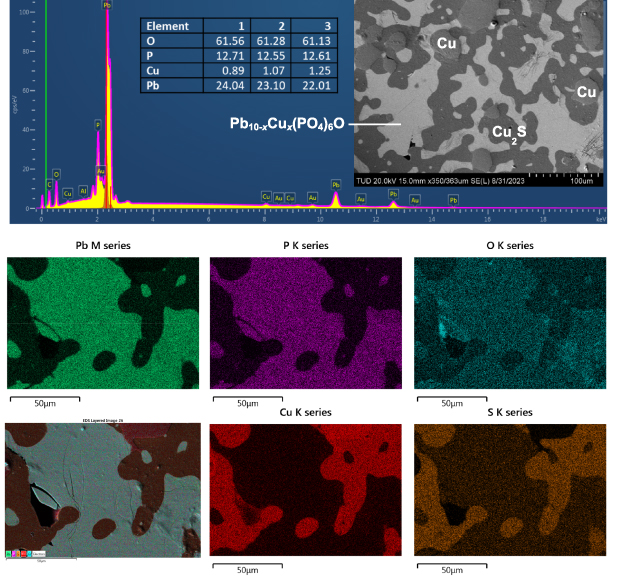
Lee et al emphasized the importance of copper-doping to achieve superconductivity in 'LK-99' [1, 2]. Hence, it seems essential to investigate the actual amount x of copper in Pb10−x Cux (PO4)6O. We performed semi-quantitative elemental analysis using SEM-EDX on all Pb10(PO4)6O type phases we obtained. All spots in the tested samples show the presence of Pb, P and O in the expected ratio. In the samples Ia and Ib, copper was detected but much less than the nominal composition Pb9Cu(PO4)6O (x = 1) would demand. SEM imaging and EDX mapping (figure 6) of a well-polished piece of sample Ib show microscopic islands of Pb10−x Cux (PO4)6O (light gray), Cu2S (dark gray) and droplets of Cu metal, indicating inhomogeneity on a microscopic scale and even inside the grains. The average value of x ∼ 0.4–0.5 was obtained from the EDX analysis. However, essentially no copper was detected inside the selected crystals of Pb10(PO4)6O obtained by route III.
Figure 6. Top: Representative EDX spectrum and the secondary electron image of a region of sample obtained from synthetic route Ib. Peaks for Al and C arise respectively from the sample holder and carbon tape used to mount the sample. Below: EDX mapping on a smaller area indicating the dark grey regions only contain Cu and S (pertaining to Cu2S) and the lighter regions corresponds to Pb10−x Cux (PO4)6O phase.
Download figure:
Standard image High-resolution imageSurprisingly most of the reports on LK-99 replication [4, 5, 7–10, 12, 14–16, 20] lack any compositional analysis. Puphal et al, succeeded in growing large single crystals of Pb10−x Cux (PO4)6O using an optical floating zone method with a varying range of 'x' as determined by EDX and diffraction analysis [11]. The samples were clearly transparent and hence, insulating. Interestingly, the copper concentration varied along the length of the crystal (with x = 0.4–1.2), which indicates inhomogeneous nature of the Cu-doping. Liu et al [13] analyzed various polycrystalline samples in detail and found the value of 'x' ranging from 0.4 to 0.7 in different regions along with copper-rich phases.
It is clear from this and previous studies that it is difficult to substitute Pb for Cu with x ≈ 1, counter to the claim by Lee et al. Chemical intuition also supports this conjecture, as the ionic radii [21] and coordination preference of Pb2+ and Cu2+ cations is significantly different, as well is their chemical behavior. Although Lee et al emphasize that substantial Cu-doping is crucial for superconductivity in 'LK-99', they have not presented any quantitative elemental analysis that could reveal the correct stoichiometry of the superconducting compound. Shrinkage of the lattice parameter was taken as the only evidence of Cu-doping (see comment on absorption effects above). Pb10−x Cux (PO4)6O with x ≪ 1, however, should be insulating and diamagnetic, which other authors also observed.
3.2. Magnetic half-levitation and properties
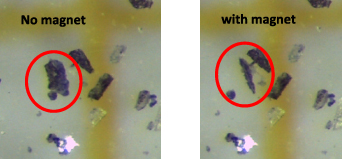
The samples I–III, which contained the purported superconducting 'LK-99' phase, were tested for magnetic levitation as a proof of strong diamagnetism associated with superconductivity. Small chunks were placed on a glass petri dish over a strong neodymium-iron-boride magnet. No visible levitation or repulsion was observed, except for few tiny flakes in samples I, II and VII that visibly responded to the permanent magnet. These flakes were not attracted by the magnet, but aligned themselves perpendicular to the surface and always tilted in the direction opposite to the magnet's movements (possibly following the magnetic field lines). This gave an impression of 'half-levitation' of the particles (figure 7). Similar behavior was also recently reported by Wu et al [9], who misinterpreted it as magnetic levitation due to superconductivity. Unfortunately, these authors performed no elemental analysis on their particles. Our analysis of the moving particles revealed that they are Pb10−x Cux (PO4)6O phase, with some containing very small traces of iron on the surface while others do not. The most likely source of iron contamination is the use of metallic spatula for weighing the starting materials (the sample was scraped from mortar pestle using a plastic spatula though). Guo et al [19] observed a similar behavior in their sample, yet provided no compositional analysis for it. Nonetheless, these observations must be interpreted cautiously and should not be confused with the Meissner effect or levitation due to superconductivity without other strong experimental evidence.
Figure 7. A tiny flake of Pb10−x Cux (PO4)6O (left) aligns vertically (right) after placing a permanent magnet underneath (at room temperature).
Download figure:
Standard image High-resolution imageWe collected magnetic data on three samples using temperature-dependent and isothermal magnetization measurements. All the samples were found to be diamagnetic with no perceptible temperature dependence (figure 8), divergence in zfc/fc data or a phase transition in the measured temperature range. This largely excludes the presence of superconductivity. We found a hysteresis at low-temperature (figures 8(b) and (f)) indicating a weak and soft ferromagnetic-like behavior. Even at 325 K, this behavior persists but is superimposed by the strong diamagnetic response from the sample. Guo et al [19] observed similar features in their sample, and Kumar et al [4] also obtained a similar hysteresis curve at 280 K in their first sample. This is intriguing as no 'ferromagnetic' elements are present in the sample and due care was taken not to use any metal spatula for weighing or scraping the sample powder while preparation of sample Ia and Ib. Remarkably, the magnitude of the saturation magnetization determined by Jia et al (∼5.5 × 10–2 emu g–1 at 100 and 300 K) almost coincides with that of our sample Ia (∼5.7 × 10–2 emu g–1), making the presence of a magnetic impurity unlikely. However, in our and the samples of other authors there are a lot of side phases that influence the overall magnetic behavior and even surface or interface effects can contribute. Similar magnetic effects were reported by Puphal et al [11] for their single-crystals, which strongly suggests that weak soft ferromagnetic behavior is intrinsically associated with Pb10−x Cux (PO4)6O. Puphal et al [11] have suggested that it could arise from copper-rich clusters within the structure. The origin of the weak ferromagnetism and to what extend it is related to the half-levitation remains unclear.
Figure 8. (a), (c), (e) Temperature dependent and (b), (d), (f) isothermal magnetization for three Pb10−x Cux (PO4)6O samples from syntheses Ia, Ib, and III respectively.
Download figure:
Standard image High-resolution image4. Conclusions and outlook
Based on our experimental results, we conclude that it is rather challenging to achieve Pb9Cu(PO4)6O, also known as 'LK-99' in the way described by Lee et al. Other synthetic approaches aimed at avoiding Cu2S as a byproduct also failed. All samples of Pb10−x Cux (PO4)6O contained only minor amounts of copper (x < 0.5), which in addition appeared to be inhomogeneously distributed. The observed difficulties in the substitution of lead(II) by copper(II) can be explained by the different sizes and coordination preferences of these cations. None of our samples was superconducting although some small particles in them showed half-levitation in a magnetic field, which might be associated with a yet unexplained weak and soft ferromagnetism. Similarly, none of the currently available literature reports the reproduction of a superconducting 'LK-99' sample. Instead, the observed diamagnetism, the metallic resistivity and the phase transition at 380 K observed by Lee et al can be well attributed to Cu2S formed during the synthesis of 'LK-99' (Pb10(PO4)6O is a diamagnetic insulator) [7]. The conductivity of Cu2S has been reported in the range of 5–140 S cm–1 [22–24]. Further assessment by P K Jain also corroborated the phase transitions of Cu2S matching with that of Lee's LK-99 sample [25]. With all the current experimental evidences it appears that the LK-99 phase is not a superconductor [26]. However, a conclusive assessment is made difficult by the fact that in very few cases a chemical analysis of the samples examined was carried out. As Lee et al stressed that a sufficiently high degree of copper substitution is essential for observing RTSC, the absence of superconductivity in our (and other's) samples might actually be related to insufficient (and non-homogenous) copper content. Alternative sample preparation methods, such as high-pressure techniques or hydrothermal growth, might stabilize 'Pb9Cu(PO4)6O'.
Acknowledgments
We acknowledge financial support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through the Collaborative Research Center SFB 1143 (Project No. 247310070) and the Würzburg-Dresden Cluster of Excellence ct.qmat (EXC 2147, Project No. 39085490)
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).