Abstract
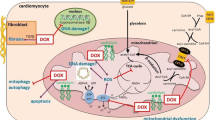
Doxorubicin (DOX) is commonly used for the treatment of various types of cancer, however can cause serious side effects, including cardiotoxicity. The mechanisms involved in DOX-induced cardiac damage are complex and not yet fully understood. One mechanism is the disruption of cardiac metabolism, which can impair cardiac function. The mammalian target of rapamycin (mTOR) is a key regulator of cardiac energy metabolism, and dysregulation of mTOR signaling has been implicated in DOX-induced cardiac dysfunction. Natural compounds (NCs) have been shown to improve cardiac function in vivo and in vitro models of DOX-induced cardiotoxicity. This review article explores the protective effects of NCs against DOX-induced cardiac injury, with a focus on their regulation of mTOR signaling pathways. Generally, the modulation of mTOR signaling by NCs represents a promising strategy for decreasing the cardiotoxic effects of DOX.


Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- β-lap:
-
Beta-LAPachone
- ADP:
-
Adenosine diphosphate
- AMPK:
-
Adenosine monophosphate–activated protein kinase
- AP:
-
Apigenin
- API:
-
Apigenin
- APS:
-
Astragalus polysaccharide
- ASP:
-
Aspalathin
- ATP:
-
Adenosine triphosphate
- BAX:
-
BCL-2-associated X protein
- BCL-2:
-
B-cell lymphoma 2
- CRA:
-
Corosolic acid
- CUR:
-
Curcumin
- Deptor:
-
DEP domain-containing mTOR-interacting protein
- DHM:
-
Dihydromyricetin
- DHT:
-
Dihydrotanshinone I
- DOX:
-
Doxorubicin
- E2F1:
-
E2 promoter binding factor 1
- ETC:
-
Electron transport chain
- FA:
-
Ferulic acid
- GL:
-
Glycyrrhizin
- HMGB1:
-
High-mobility group box 1
- LAMP1:
-
Lysosomal-associated membrane proteins-1
- LC3:
-
Protein light chain 3
- LKB1:
-
Liver kinase B1
- LUTG:
-
Luteolin-7-O-glucoside
- mLST8:
-
Mammalian lethal with SEC13 protein 8
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
MTOR complex 1
- mTORC2:
-
MTOR complex 2
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NCs:
-
Natural compounds
- NEF:
-
Neferine
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- p-AKT:
-
Phosphorylated-AKT
- p-mTOR:
-
Phosphorylated-mTOR
- PCr:
-
Creatine phosphate
- PI3K:
-
Phosphoinositide 3-kinases
- PKC:
-
Protein kinase C
- Rags:
-
Ras-related GTP binding proteins
- Raptor:
-
Regulatory-associated protein of mTOR
- Rictor:
-
Rapamycin-insensitive companion of mTOR
- ROS:
-
Reactive oxygen species
- RSV:
-
Resveratrol
- SCU:
-
Scutellarin
- Sin1:
-
Stress-activated map kinase-interacting protein 1
- SIRT1:
-
Sirtuin 1
- SP:
-
Spinacetin
- t-AKT:
-
Total-AKT
- t-mTOR:
-
Total-mTOR
- Tan-IIA:
-
Tanshinone IIA
- TFEB:
-
Transcription factor EB
- TQ:
-
Thymoquinone
- TSC2:
-
Tuberous sclerosis complex 2
- ULK1:
-
Unc-51-like kinase 1
- WWGPE:
-
Whole wheat grain polyphenolic extract
References
Yarmohmmadi, F., Rahimi, N., Faghir-Ghanesefat, H., Javadian, N., Abdollahi, A., Pasalar, P., & Dehpour, A. R. (2017). Protective effects of agmatine on doxorubicin-induced chronic cardiotoxicity in rat. European Journal of Pharmacology, 796, 39–44. https://doi.org/10.1016/j.ejphar.2016.12.022
Okpara, E. S., Adedara, I. A., Guo, X., Klos, M. L., Farombi, E. O., & Han, S. (2022). Molecular mechanisms associated with the chemoprotective role of protocatechuic acid and its potential benefits in the amelioration of doxorubicin-induced cardiotoxicity: A review. Toxicology Reports, 9, 1713–1724. https://doi.org/10.1016/j.toxrep.2022.09.001
Rawat, P. S., Jaiswal, A., Khurana, A., Bhatti, J. S., & Navik, U. (2021). Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 139, 111708. https://doi.org/10.1016/j.biopha.2021.111708
Oxidative medicine and cellular longevity, 2022, 7176282. https://doi.org/10.1155/2022/7176282.
Journal of molecular and cellular cardiology, 69, 4–16. https://doi.org/10.1016/j.yjmcc.2014.01.007.
Szwed, A., Kim, E., & Jacinto, E. (2021). Regulation and metabolic functions of mTORC1 and mTORC2. Physiological Reviews, 101(3), 1371–1426. https://doi.org/10.1152/physrev.00026.2020
Jiao, Y., Li, Y., Zhang, J., Zhang, S., Zha, Y., & Wang, J. (2022). RRM2 alleviates doxorubicin-induced cardiotoxicity through the AKT/mTOR signaling pathway. Biomolecules, 12(2), 299. https://doi.org/10.3390/biom12020299
Molecular medicine reports, 23(4), 1–11. https://doi.org/10.3892/mmr.2021.11938.
Johnson, R., Shabalala, S., Louw, J., Kappo, A. P., & Muller, C. J. F. (2017). Aspalathin reverts Doxorubicin-Induced cardiotoxicity through increased autophagy and decreased expression of p53/mTOR/p62 signaling. Molecules (Basel Switzerland), 22(10), 1589. https://doi.org/10.3390/molecules22101589
Yarmohammadi, F., Rezaee, R., & Karimi, G. (2021). Natural compounds against doxorubicin-induced cardiotoxicity: A review on the involvement of Nrf2/ARE signaling pathway. Phytotherapy Research, 35(3), 1163–1175. https://doi.org/10.1002/ptr.6882
Lv, X., Zhu, Y., Deng, Y., Zhang, S., Zhang, Q., Zhao, B., & Li, G. (2020). Glycyrrhizin improved autophagy flux via HMGB1-dependent Akt/mTOR signaling pathway to prevent doxorubicin-induced cardiotoxicity. Toxicology, 441, 152508. https://doi.org/10.1016/j.tox.2020.152508
Phytomedicine: international journal of phytotherapy and phytopharmacology, 99, 154027. https://doi.org/10.1016/j.phymed.2022.154027.
Manolis, A. S., Manolis, A. A., Manolis, T. A., Apostolaki, N. E., Apostolopoulos, E. J., Melita, H., & Katsiki, N. (2021). Mitochondrial dysfunction in cardiovascular disease: Current status of translational research/clinical and therapeutic implications. Medicinal Research Reviews, 41(1), 275–313. https://doi.org/10.1002/med.21732
Garcia-Ropero, A., Santos-Gallego, C. G., Zafar, M. U., & Badimon, J. J. (2019). Metabolism of the failing heart and the impact of SGLT2 inhibitors. Expert Opinion on drug Metabolism & Toxicology, 15(4), 275–285. https://doi.org/10.1080/17425255.2019.1588886
Scientific Reports, 13(1), 8346. https://doi.org/10.21203/rs.3.rs-614231/v1.
Carvalho, R. A., Sousa, R. P. B., Cadete, V. J. J., Lopaschuk, G. D., Palmeira, C. M. M., Bjork, J. A., & Wallace, K. B. (2010). Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology, 270(2), 92–98. https://doi.org/10.1016/j.tox.2010.01.019
Journal of applied toxicology: JAT, 36(11), 1486–1495. https://doi.org/10.1002/jat.3307.
γ‐mediated metabolic reprogramming in cardiomyocytes. The Journal of pathology, 247(3), 320–332. https://doi.org/10.1002/path.5192.
Gorini, S., De Angelis, A., Berrino, L., Malara, N., Rosano, G., & Ferraro, E. (2018). Chemotherapeutic drugs and mitochondrial dysfunction: Focus on doxorubicin, trastuzumab, and sunitinib. Oxidative medicine and cellular longevity, 2018, 7582730. https://doi.org/10.1155/2018/7582730
Nolfi-Donegan, D., Braganza, A., & Shiva, S. (2020). Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biology, 37, 101674. https://doi.org/10.1016/j.redox.2020.101674
Russo, M., Della Sala, A., Tocchetti, C. G., Porporato, P. E., & Ghigo, A. (2021). Metabolic aspects of anthracycline cardiotoxicity. Current Treatment Options in Oncology, 22(2), 18. https://doi.org/10.1007/s11864-020-00812-1
Shi, S., Chen, Y., Luo, Z., Nie, G., & Dai, Y. (2023). Role of oxidative stress and inflammation-related signaling pathways in doxorubicin-induced cardiomyopathy. Cell Communication and Signaling: CCS, 21(1), 61. https://doi.org/10.1186/s12964-023-01077-5
Nature cancer, 1(3), 315–328. https://doi.org/10.1038/s43018-020-0039-1.
Chen, Y., & Zhou, X. (2020). Research progress of mTOR inhibitors. European Journal of Medicinal Chemistry, 208, 112820. https://doi.org/10.1016/j.ejmech.2020.112820
Getz, L. J., Runte, C. S., Rainey, J. K., & Thomas, N. A. (2019). Tyrosine phosphorylation as a widespread regulatory mechanism in prokaryotes. Journal of Bacteriology, 201(19), e00205–e00219. https://doi.org/10.1128/JB.00205-19
Sciarretta, S., Forte, M., Frati, G., & Sadoshima, J. (2018). New insights into the role of mTOR signaling in the cardiovascular system. Circulation Research, 122, 489–505. https://doi.org/10.1161/CIRCRESAHA.117.311147
Mao, Z., & Zhang, W. (2018). Role of mTOR in glucose and lipid metabolism. International Journal of Molecular Sciences, 19(7). https://doi.org/10.3390/ijms19072043
Molecular cell, 39(2), 171–183. https://doi.org/10.1016/j.molcel.2010.06.022.
Shi, F., & Collins, S. (2023). Regulation of mTOR signaling: Emerging role of cyclic nucleotide-dependent protein kinases and implications for cardiometabolic disease. International Journal of Molecular Sciences, 24(14). https://doi.org/10.3390/ijms241411497
Kaldirim, M., Lang, A., Pfeiler, S., Fiegenbaum, P., Kelm, M., Bönner, F., & Gerdes, N. (2022). Modulation of mTOR signaling in cardiovascular disease to target acute and chronic inflammation. Frontiers in Cardiovascular Medicine, 9, 907348. https://doi.org/10.3389/fcvm.2022.907348
Aging Cell, 19(4), e13126. https://doi.org/10.1111/acel.13126.
Science (New York, N.Y.), 366(6464), 468–475. https://doi.org/10.1126/science.aay0166.
Nature, 614(7948), 572–579. https://doi.org/10.1038/s41586-022-05652-7.
Dang, T. T., & Back, S. H. (2021). Translation inhibitors activate autophagy master regulators TFEB and TFE3. International Journal of Molecular Sciences, 22(21), 12083. https://doi.org/10.3390/ijms222112083
Deleyto-Seldas, N., & Efeyan, A. (2021). The mTOR–autophagy axis and the control of metabolism. Frontiers in Cell and Developmental Biology, 9, 655731. https://doi.org/10.3389/fcell.2021.655731
Sadria, M., & Layton, A. T. (2021). Interactions among mTORC, AMPK and SIRT: A computational model for cell energy balance and metabolism. Cell Communication and Signaling, 19(1), 1–17. https://doi.org/10.1101/2020.10.07.330308
Bazer, F. W., Seo, H., Wu, G., & Johnson, G. A. (2020). Interferon tau: Influences on growth and development of the conceptus. Theriogenology, 150, 75–83. https://doi.org/10.1016/j.theriogenology.2020.01.069
Jacinto, E. (2019). Amplifying mTORC2 signals through AMPK during energetic stress. Science Signaling, 12(585), eaax5855. https://doi.org/10.1126/scisignal.aax5855
Fu, W., & Hall, M. N. (2020). Regulation of mTORC2 signaling. Genes, 11(9), 1045. https://doi.org/10.3390/genes11091045
Nature Communications, 11(1), 575. https://doi.org/10.1038/s41467-020-14430-w.
Journal of Diabetes Research, 2022. https://doi.org/10.1155/2022/1755563.
Shi, B., Ma, M., Zheng, Y., Pan, Y., & Lin, X. (2019). mTOR and Beclin1: Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. Journal of Cellular Physiology, 234(8), 12562–12568. https://doi.org/10.1002/jcp.28125
Circulation research, 132(5), e148–e165. https://doi.org/10.1161/CIRCRESAHA.119.316388.
Daneshgar, N., Rabinovitch, P. S., & Dai, D. F. (2021). TOR signaling pathway in cardiac aging and heart failure. Biomolecules, 11(2), 168. https://doi.org/10.3390/biom11020168
Mohan, U. P., PB, T. P., Iqbal, S. T. A., & Arunachalam, S. (2021). Mechanisms of doxorubicin-mediated reproductive toxicity–a review. Reproductive Toxicology, 102, 80–89. https://doi.org/10.1016/j.reprotox.2021.04.003
Oncotarget, 8(3), 4837–4848. https://doi.org/10.18632/oncotarget.13596.
β-LAPachone ameliorates doxorubicin-induced cardiotoxicity via regulating autophagy and Nrf2 signalling pathways in mice. Basic & clinical pharmacology & toxicology, 126(4), 364–373. https://doi.org/10.1111/bcpt.13340.
Biochemical Pharmacology, 180, 114188. https://doi.org/10.1016/j.bcp.2020.114188.
Yu, W., Qin, X., Zhang, Y., Qiu, P., Wang, L., Zha, W., & Ren, J. (2020). Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovascular Diagnosis and Therapy, 10(4), 752. https://doi.org/10.21037/cdt-19-707
Wang, M., Firrman, J., Liu, L., & Yam, K. (2019). A review on flavonoid apigenin: Dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. BioMed Research International, 2019, 7010467. https://doi.org/10.1155/2019/7010467
Thomas, S. D., Jha, N. K., Jha, S. K., Sadek, B., & Ojha, S. (2023). Pharmacological and molecular insight on the cardioprotective role of apigenin. Nutrients, 15(2), 385. https://doi.org/10.3390/nu15020385
Yu, W., Sun, H., Zha, W., Cui, W., Xu, L., Min, Q., & Wu, J. (2017). Apigenin attenuates adriamycin-induced cardiomyocyte apoptosis via the PI3K/AKT/mTOR pathway. Evidence-based complementary and alternative medicine: eCAM, 2017, 2590676. https://doi.org/10.1155/2017/2590676
Biomolecules, 11(3), 392. https://doi.org/10.3390/biom11030392.
Fu, Y. S., Chen, T. H., Weng, L., Huang, L., Lai, D., & Weng, C. F. (2021). Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomedicine & Pharmacotherapy, 141, 111888. https://doi.org/10.1016/j.biopha.2021.111888
Vafaeipour, Z., Razavi, B. M., & Hosseinzadeh, H. (2022). Effects of turmeric (Curcuma longa) and its constituent (curcumin) on the metabolic syndrome: An updated review. Journal of Integrative Medicine, 20(3), 193–203. https://doi.org/10.1039/D2FO02625B
Yarmohammadi, F., Hayes, A. W., & Karimi, G. (2021). Protective effects of curcumin on chemical and drug-induced cardiotoxicity: A review. Naunyn-Schmiedeberg’s Archives of Pharmacology, 394, 1341–1353. https://doi.org/10.1007/s00210-021-02072-8
Psychopharmacology, 240(5), 1179–1190. https://doi.org/10.1007/s00213-023-06357-z.
Zhang, Q., & Wu, L. (2022). In vitro and in vivo cardioprotective effects of curcumin against doxorubicin-induced cardiotoxicity: A systematic review. Journal of oncology, 2022, 7277562. https://doi.org/10.1155/2022/7277562
Yao, H., Zhou, L., Tang, L., Guan, Y., Chen, S., Zhang, Y., & Han, X. (2017). Protective effects of luteolin-7-O-glucoside against starvation-induced injury through upregulation of autophagy in H9c2 cells. Bioscience Trends, 11(5), 557–564. https://doi.org/10.5582/bst.2017.01111
Zang, Y., Igarashi, K., & Li, Y. (2016). Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A y mice. Bioscience Biotechnology and Biochemistry, 80(8), 1580–1586. https://doi.org/10.1080/09168451.2015.1116928
Nutrients, 14(6), 1155. https://doi.org/10.3390/nu14061155.
Biomolecules, 10(4), 502. https://doi.org/10.3390/biom10040502.
Cardiovascular toxicology, 16(2), 101–110. https://doi.org/10.1007/s12012-015-9317-z.
Biomedicine & Pharmacotherapy, 158, 114203. https://doi.org/10.1016/j.biopha.2022.114203.
Asokan, S. M., Mariappan, R., Muthusamy, S., & Velmurugan, B. K. (2018). Pharmacological benefits of neferine-A comprehensive review. Life Sciences, 199, 60–70. https://doi.org/10.1016/j.lfs.2018.02.032
Guolan, D., Lingli, W., Wenyi, H., Wei, Z., Baowei, C., & Sen, B. (2018). Anti-inflammatory effects of neferine on LPS-induced human endothelium via MAPK, and NF-κβ pathways. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 73(9), 541–544. https://doi.org/10.1691/ph.2018.8443
Lohanathan, B. P., Rathinasamy, B., Huang, C. Y., & Viswanadha, V. P. (2022). Neferine attenuates doxorubicin-induced fibrosis and hypertrophy in H9c2 cells. Journal of Biochemical and Molecular Toxicology, 36(7), e23054. https://doi.org/10.1002/jbt.23054
Bharathi Priya, L., Baskaran, R., Huang, C. Y., & Vijaya Padma, V. (2018). Neferine modulates IGF-1R/Nrf2 signaling in doxorubicin treated H9c2 cardiomyoblasts. Journal of Cellular Biochemistry, 119(2), 1441–1452. https://doi.org/10.1002/jcb.26305
Van Hung, P. (2016). Phenolic compounds of cereals and their antioxidant capacity. Critical Reviews in Food Science and Nutrition, 56(1), 25–35. https://doi.org/10.1080/10408398.2012.708909
Ma, D., Wang, C., Feng, J., & Xu, B. (2021). Wheat grain phenolics: A review on composition, bioactivity, and influencing factors. Journal of the Science of Food and Agriculture, 101(15), 6167–6185. https://doi.org/10.1002/jsfa.11428
Sahu, R., Dua, T. K., Das, S., De Feo, V., & Dewanjee, S. (2019). Wheat phenolics suppress doxorubicin-induced cardiotoxicity via inhibition of oxidative stress, MAP kinase activation, NF-κB pathway, PI3K/Akt/mTOR impairment, and cardiac apoptosis. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 125, 503–519. https://doi.org/10.1016/j.fct.2019.01.034
Zhao, J., Zhou, H., An, Y., Shen, K., & Yu, L. (2021). Biological effects of corosolic acid as an anti–inflammatory, anti–metabolic syndrome and anti–neoplasic natural compound. Oncology Letters, 21(2), 1. https://doi.org/10.3892/ol.2020.12345
International Journal of Molecular Medicine, 45(5), 1425–1435. https://doi.org/10.3892/ijmm.2020.4531.
Alkholifi, F. K., Devi, S., Yusufoglu, H. S., & Alam, A. (2023). The cardioprotective effect of corosolic acid in the diabetic rats: A possible mechanism of the PPAR-γ pathway. Molecules, 28(3), 929. https://doi.org/10.3390/molecules28030929
Che, Y., Wang, Z., Yuan, Y., Zhou, H., Wu, H., Wang, S., & Tang, Q. (2022). By restoring autophagic flux and improving mitochondrial function, corosolic acid protects against dox-induced cardiotoxicity. Cell Biology and Toxicology, 38(3), 451–467. https://doi.org/10.1007/s10565-021-09619-8
International Journal of Molecular Sciences, 23(23), 15180. https://doi.org/10.3390/ijms232315180.
Wei, Y., Xu, M., Ren, Y., Lu, G., Xu, Y., Song, Y., & Ji, H. (2016). The cardioprotection of dihydrotanshinone I against myocardial ischemia–reperfusion injury via inhibition of arachidonic acid ω-hydroxylase. Canadian Journal of Physiology and Pharmacology, 94(12), 1267–1275. https://doi.org/10.1139/cjpp-2016-0036
κB inflammatory signaling axis: a novel therapeutic pathway of dihydrotanshinone I in doxorubicin-induced cardiotoxicity. Journal of experimental & clinical cancer research: CR, 39(1), 93. https://doi.org/10.1186/s13046-020-01595-x.
Yarmohammadi, F., Karbasforooshan, H., Hayes, A. W., & Karimi, G. (2021). Inflammation suppression in doxorubicin-induced cardiotoxicity: Natural compounds as therapeutic options. Naunyn-Schmiedeberg’s Archives of Pharmacology, 394(10), 2003–2011. https://doi.org/10.1007/s00210-021-02132-z
Fang, Z., Zhang, M., Liu, J., Zhao, X., Zhang, Y., & Fang, L. (2021). Tanshinone IIA: A review of its anticancer effects. Frontiers in Pharmacology, 11, 611087. https://doi.org/10.3389/fphar.2020.611087
Guo, R., Li, L., Su, J., Li, S., Duncan, S. E., Liu, Z., & Fan, G. (2020). Pharmacological activity and mechanism of tanshinone IIA in related diseases. Drug Design Development and Therapy, 14, 4735–4748. https://doi.org/10.2147/DDDT.S266911
Feng, J., Liu, L., Yao, F., Zhou, D., He, Y., & Wang, J. (2021). The protective effect of tanshinone IIa on endothelial cells: A generalist among clinical therapeutics. Expert Review of Clinical Pharmacology, 14(2), 239–248. https://doi.org/10.1080/17512433.2021.1878877
He, Z., Sun, C., Xu, Y., & Cheng, D. (2016). Reduction of atrial fibrillation by Tanshinone IIA in chronic heart failure. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 84, 1760–1767. https://doi.org/10.1016/j.biopha.2016.10.110
Frontiers in Pharmacology, 14, 1165212. https://doi.org/10.3389/fphar.2023.1165212.
Cancers, 11(7), 910. https://doi.org/10.3390/cancers11070910.
Chaudhary, S. K., Sandasi, M., Makolo, F., van Heerden, F. R., & Viljoen, A. M. (2021). Aspalathin: A rare dietary dihydrochalcone from aspalathus linearis (rooibos tea). Phytochemistry Reviews, 1–32. https://doi.org/10.1007/s11101-021-09741-9
Molecules, 24(9), 1713. https://doi.org/10.3390/molecules24091713.
Li, C., Liu, Y., Zhang, Y., Li, J., & Lai, J. (2022). Astragalus polysaccharide: A review of its immunomodulatory effect. Archives of Pharmacal Research, 45(6), 367–389. https://doi.org/10.1007/s12272-022-01393-3
Zheng, Y., Ren, W., Zhang, L., Zhang, Y., Liu, D., & Liu, Y. (2020). A review of the pharmacological action of Astragalus polysaccharide. Frontiers in Pharmacology, 11, 349. https://doi.org/10.3389/fphar.2020.00349
Liu, D., Chen, L., Zhao, J., & Cui, K. (2018). Cardioprotection activity and mechanism of astragalus polysaccharide in vivo and in vitro. International Journal of Biological Macromolecules, 111, 947–952. https://doi.org/10.1016/j.ijbiomac.2018.01.048
Zhang, Y., Zhou, Q., Ding, X., Wang, H., & Tan, G. (2021). HILIC-MS-based metabolomics reveal that astragalus polysaccharide alleviates doxorubicin-induced cardiomyopathy by regulating sphingolipid and glycerophospholipid homeostasis. Journal of Pharmaceutical and Biomedical Analysis, 203, 114177. https://doi.org/10.1016/j.jpba.2021.114177
Zhang, H., Caprioli, G., Hussain, H., Le, N. P. K., Farag, M. A., & Xiao, J. (2021). A multifaceted review on dihydromyricetin resources, extraction, bioavailability, biotransformation, bioactivities, and food applications with future perspectives to maximize its value. eFood, 2(4), 164–184. https://doi.org/10.53365/efood.k/143518
Frontiers in Pharmacology, 12, 3721. https://doi.org/10.3389/fphar.2021.794563.
Wang, Y., Wang, J., Xiang, H., Ding, P., Wu, T., & Ji, G. (2022). Recent update on application of dihydromyricetin in metabolic related diseases. Biomedicine & Pharmacotherapy, 148, 112771. https://doi.org/10.1016/j.biopha.2022.112771
Frontiers in pharmacology, 9, 1204. https://doi.org/10.3389/fphar.2018.01204.
Wang, L., & Ma, Q. (2018). Clinical benefits and pharmacology of scutellarin: A comprehensive review. Pharmacology & Therapeutics, 190, 105–127. https://doi.org/10.1016/j.pharmthera.2018.05.006
Huang, H., Geng, Q., Yao, H., Shen, Z., Wu, Z., Miao, X., & Shi, P. (2018). Protective effect of scutellarin on myocardial infarction induced by isoprenaline in rats. Iranian Journal of Basic Medical Sciences, 21(3), 267. https://doi.org/10.22038/ijbms.2018.26110.6415
Xu, L., Chen, R., Ma, X., Zhu, Y., Sun, G., & Sun, X. (2020). Scutellarin protects against myocardial ischemia-reperfusion injury by suppressing NLRP3 inflammasome activation. Phytomedicine, 68, 153169. https://doi.org/10.1016/j.phymed.2020.153169
Chemistry & biodiversity, 20(1), e202200450. https://doi.org/10.1002/cbdv.202200450.
Toxicology in vitro: an international journal published in association with BIBRA, 82, 105366. https://doi.org/10.1016/j.tiv.2022.105366.
Lu, Y., Feng, Y., Liu, D., Zhang, Z., Gao, K., Zhang, W., & Tang, H. (2018). Thymoquinone attenuates myocardial ischemia/reperfusion injury through activation of SIRT1 signaling. Cellular Physiology and Biochemistry, 47(3), 1193–1206. https://doi.org/10.1159/000490216
Tabassum, S., Rosli, N., Ichwan, S. J. A., & Mishra, P. (2021). Thymoquinone and its pharmacological perspective: A review. Pharmacological Research - Modern Chinese Medicine, 1, 100020. https://doi.org/10.1016/j.prmcm.2021.100020
Gur, F. M., & Aktas, I. (2021). The ameliorative effects of thymoquinone and beta-aminoisobutyric acid on streptozotocin-induced diabetic cardiomyopathy. Tissue and Cell, 71, 101582. https://doi.org/10.1016/j.tice.2021.101582
Liu, D., & Zhao, L. (2022). Thymoquinone–induced autophagy mitigates doxorubicin–induced H9c2 cell apoptosis. Experimental and Therapeutic Medicine, 24(5), 694. https://doi.org/10.3892/etm.2022.11630
Nagar, P. S., Rane, S., & Dwivedi, M. (2022). LC-MS/MS standardization and validation of glycyrrhizin from the roots of taverniera cuneifolia: A potential alternative source of glycyrrhiza glabra. Heliyon, 8(8), e10234. https://doi.org/10.1016/j.heliyon.2022.e10234
Thakur, V., Alcoreza, N., Delgado, M., Joddar, B., & Chattopadhyay, M. (2021). Cardioprotective effect of glycyrrhizin on myocardial remodeling in diabetic rats. Biomolecules, 11(4), 569. https://doi.org/10.3390/biom11040569
Translational stroke research, 11, 967–982. https://doi.org/10.1007/s12975-019-00772-1.
Mano, Y., Abe, K., Takahashi, M., Higurashi, T., Kawano, Y., Miyazaki, S., & Maeda-Minami, A. (2023). Optimal administration of glycyrrhizin avoids pharmacokinetic interactions with high-dose methotrexate and exerts a hepatoprotective effect. Anticancer Research, 43(4), 1493–1501. https://doi.org/10.21873/anticanres.16298
Jäger, T., Mokos, A., Prasianakis, N. I., & Leyer, S. (2022). Pore-level multiphase simulations of realistic distillation membranes for water desalination. Membranes, 11(4), 569. https://doi.org/10.3390/biom11040569
Naunyn-Schmiedeberg’s Archives of Pharmacology, 393, 979–989. https://doi.org/10.1007/s00210-019-01767-3.
Frontiers in Immunology, 11, 1104. https://doi.org/10.3389/fimmu.2020.01104.
κB and PI3K/Akt/mTOR pathways. Molecular immunology, 94, 7–17. https://doi.org/10.1016/j.molimm.2017.12.008.
Oh, H., Choi, A., Seo, N., Lim, J. S., You, J. S., & Chung, Y. E. (2021). Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on post-contrast acute kidney injury. Scientific Reports, 11(1), 1–12. https://doi.org/10.1038/s41598-021-94928-5
Malviya, V., Tawar, M., Burange, P., & Jodh, R. (2022). A brief review on resveratrol, 12(2), 157–162. https://doi.org/10.52711/2231-5659.2022.00027
Dyck, G. J. B., Raj, P., Zieroth, S., Dyck, J. R. B., & Ezekowitz, J. A. (2019). The effects of resveratrol in patients with cardiovascular disease and heart failure: A narrative review. International Journal of Molecular Sciences, 20(4), 904. https://doi.org/10.3390/ijms20040904
Li, T., Tan, Y., Ouyang, S., He, J., & Liu, L. (2022). Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene, 808, 145968. https://doi.org/10.1016/j.gene.2021.145968
Gu, J., Fan, Y. Q., Zhang, H. L., Pan, J. A., Yu, J. Y., Zhang, J. F., & Wang, C. Q. (2018). Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1 mediated autophagy inhibition and apoptosis promotion. Biochemical Pharmacology, 150, 202–213. https://doi.org/10.1016/j.bcp.2018.02.025
Gu, J., Hu, W., Song, Z. P., Chen, Y. G., Zhang, D. D., & Wang, C. Q. (2016). Resveratrol-induced autophagy promotes survival and attenuates doxorubicin-induced cardiotoxicity. International Immunopharmacology, 32, 1–7. https://doi.org/10.1016/j.intimp.2016.01.002
Lei, Y., & Klionsky, D. J. (2023). Transcriptional regulation of autophagy and its implications in human disease. Cell Death & Differentiation, 1–14. https://doi.org/10.1038/s41418-023-01162-9
Rahman, M. A., Cho, Y., Nam, G., & Rhim, H. (2021). Antioxidant compound, oxyresveratrol, inhibits APP production through the AMPK/ULK1/mTOR-mediated autophagy pathway in mouse cortical astrocytes. Antioxidants, 10(3), 408. https://doi.org/10.3390/antiox10030408
Gomes, C. L., de Albuquerque Wanderley Sales, V., Gomes de Melo, C., Ferreira da Silva, R. M., Nishimura, V., Rolim, R. H., L. A., & Rolim Neto, P. J. (2021). Beta-lapachone: Natural occurrence, physicochemical properties, biological activities, toxicity and synthesis. Phytochemistry, 186, 112713. https://doi.org/10.1016/j.phytochem.2021.112713
β-Lapachone protects against doxorubicin-induced nephrotoxicity via NAD+/AMPK/NF-kB in mice. Naunyn-Schmiedeberg’s Archives of Pharmacology, 392, 633–640. https://doi.org/10.1007/s00210-019-01619-0.
Tabrizi, F. B., Yarmohammadi, F., Hayes, A. W., & Karimi, G. (2022). The modulation of SIRT1 and SIRT3 by natural compounds as a therapeutic target in doxorubicin-induced cardiotoxicity: A review. Journal of Biochemical and Molecular Toxicology, 36(1), e22946. https://doi.org/10.1002/jbt.22946
Chemico-biological interactions, 317, 108972. https://doi.org/10.1016/j.cbi.2020.108972.
Human Gene Therapy, 33(11–12), 598–613. https://doi.org/10.1089/hum.2021.176.
Frontiers in Pharmacology, 13, 870699. https://doi.org/10.3389/fphar.2022.870699.
Liu, D., & Zhao, L. (2022). Spinacetin alleviates doxorubicin-induced cardiotoxicity by initiating protective autophagy through SIRT3/AMPK/mTOR pathways. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 101, 154098. https://doi.org/10.1016/j.phymed.2022.154098
Frontiers in Pharmacology, 9, 824. https://doi.org/10.3389/fphar.2018.00824.
Murcia, M. A., Jiménez-Monreal, A. M., Gonzalez, J., & Martínez-Tomé, M. (2020). Chapter 11 - Spinach. In A. K. B. T.-N. C. and A. P. of F. and V. Jaiswal (Ed.), (pp. 181–195). Academic Press. https://doi.org/10.1016/B978-0-12-812780-3.00011-8
Zhang, T., Liu, J., Tong, Q., & Lin, L. (2020). SIRT3 acts as a positive autophagy regulator to promote lipid mobilization in adipocytes via activating AMPK. International Journal of Molecular Sciences, 21(2), 372. https://doi.org/10.3390/ijms21020372
Acknowledgements
Authors are grateful to the Kermanshah University of Medical Sciences Office of Vice Chancellor for Research, Kermanshah, Iran.
Author information
Authors and Affiliations
Contributions
F.Y. and M.H. wrote the main manuscript text. F.Y. prepared figures. D.SH. edited the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All the authors are ready to publish the manuscript in ‘Cardiovascular Toxicology’ as per rule and regulations of the journal.
Competing Interests
The authors declare no competing interests.
Additional information
Communicated by Yajing Wang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yarmohammadi, F., Hesari, M. & Shackebaei, D. The Role of mTOR in Doxorubicin-Altered Cardiac Metabolism: A Promising Therapeutic Target of Natural Compounds. Cardiovasc Toxicol 24, 146–157 (2024). https://doi.org/10.1007/s12012-023-09820-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-023-09820-7