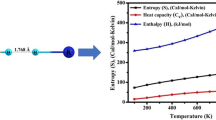
Abstract
A theoretical study was conducted to investigate the dihydrogen and alkali-halogen bonding in binary X3CH⋅⋅⋅HNa, X2CH2⋅⋅⋅HNa and ternary complexes 2(X3CH)⋅⋅⋅HNa, 2(X2CH2)⋅⋅⋅HNa (where X = F, Cl, Br). The computations were performed using the B3LYP method with different basis sets, namely pople’s (6-311++G**) and dunning type (aug-cc-pVDZ and aug-cc-pVTZ). Additionally, dispersion-corrected density functional theory calculations were carried out for all the structures. The interpretation of structural parameters through interaction energy revealed that Br3CH⋅⋅⋅HNa complex has the shortest binding distance with more interaction energy. The results illustrate that the H⋅⋅⋅H interaction is strengthened in the ternary complexes compared to binary. The vibrational analysis divulged that C–H and H–Na stretching frequencies are blue and red shifted upon dihydrogen bond formation. Moreover, natural bond orbital (NBO), quantum theory of atoms in molecule (QTAIM), non-covalent interaction (NCI)–reduced density gradient (RDG) analysis were carried out to understand the nature of intermolecular interactions, followed by the molecular electrostatic potential (MEP) analysis which confirm the existence of non-covalent interaction between C‒H and H–Na bonds.




Similar content being viewed by others
REFERENCES
S. Amiri, A. Zabardasti, and S. Farhadi, Chem. Pap. 73, 1447 (2019).
Y. Li, L. Zhang, S. Du, F. de Ren, and W. Liang Wang, Comput. Theor. Chem. 977, 201 (2011).
B. G. Oliveira, C. R. Chim. 19, 995 (2016).
P. D. Duraisamy, P. Gopalan, and A. Angamuthu, Chem. Pap. 74, 1609 (2020).
J. C. Lee, E. Peris, A. L. Rheingold, and R. H. Crabtree, J. Am. Chem. Soc. 116, 11014 (1994).
A. J. Lough, S. Park, R. Ramachandran, and R. H. Morris, J. Am. Chem. Soc. 116, 8356 (1994).
R. Custelcean and J. E. Jackson, Chem. Rev. 101, 1963 (2001).
I. Alkorta, J. De, V. Cier, M. Ya, and J. E. Del Bene, J. Phys. Chem. A 106, 9325 (2002).
J. G. Planas, C. Viñas, F. Teixidor, A. Comas-Vives, G. Ujaque, and A. Lledós, J. Am. Chem. Soc. 127, 15976 (2005).
K. Verma and K. S. Viswanathan, Phys. Chem. Chem. Phys. 19, 19067 (2017).
G. N. Patwari, T. Ebata, and N. Mikami, J. Chem. Phys. 114, 8877 (2001).
P. Lipkowski, S. J. Grabowski, T. L. Robinson, and J. Leszczynski, J. Phys. Chem. A 108, 10865 (2004).
W. Zierkiewicz and P. Hobza, Phys. Chem. Chem. Phys. 6, 5288 (2004).
P. D. Duraisamy, P. Gopalan, and A. Angamuthu, Monatsh. Chem. 151, 1569 (2020).
B. G. Oliveira, Comput. Theor. Chem. 998, 173 (2012).
C. R. Fuson and B. A. Bull, Chem. Rev. 15, 3 (1934).
H. Qian, Y. L. Lin, B. Xu, L. P. Wang, Z. C. Gao, and N. Y. Gao, Chem. Eng. J. 349, 849 (2018).
H. L. Hassinger, R. M. Soll, and G. W. Gribble, Tetrahedron Lett. 39, 3095 (1998).
J. Friedrich, S. Wettmarshausen, and M. Hennecke, Surf. Coat. Technol. 203, 3647 (2009).
S. Saravanan, V. Nagarajan, A. Srivastava, and R. Chandiramouli, Int. J. Environ. Anal. Chem., 0306 (2019).
M. Protocol, C. Cao, Y. Chen, Y. Wu, and E. Deumens, Int. J. Quantum Chem. 111, 4020 (2011).
B. Hui Li, W. Jing Shi, and F. de Ren, Comput. Theor. Chem. 1020, 81 (2013).
E. Cubero, M. Orozco, and F. J. Luque, Chem. Phys. Lett. 310, 445 (1999).
B. G. Oliveira, Comput. Theor. Chem. 998, 173 (2012).
Y. Mao, P. R. Horn, N. Mardirossian, T. Head-Gordon, C. K. Skylaris, and M. Head-Gordon, J. Chem. Phys. 145 (2016).
C. Lee, C. Hill, and N. Carolina, Chem. Phys. Lett. 162, 165 (1989).
A. D. Becke, J. Chem. Phys. 104, 1040 (1996).
S. F. Boys and F. Bernardi, Mol. Phys. 19, 553 (1970).
A. E. Reed, L. A. Curtiss, and F. Weinhold, Chem. Rev. 88, 899 (1988).
R. F. W. Bader, Chem. Rev. 91, 893 (1991).
T. Lu and F. Chen, J. Comput. Chem. 33, 580 (2012).
W. Humphrey, A. Dalke, and K. Schulten, J. Mol. Graph. 14, 33 (1996).
G. A. Zhurko, ChemCraft, Vers. 1.8. http://www.chemcraftprog.com.
M. J. Frisch et al., Gaussian 09, Revision B.01 (Gaussian Inc., Wallingford, CT, 2009).
ACKNOWLEDGMENTS
The authors gratefully thankful to “Bioinformatics resources and applications facility (BRAF), C-DAC, Pune” for providing the computational facilities for this work. Also, acknowledge the offering workstation from Computer Technology Centre (CTC) at KITS.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there are no conflicts of interests.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
devi Duraisamy, P., Prince Makarios Paul, S., Gopalan, P. et al. Investigation of Dihydrogen Bonded Interaction in X3CH⋅⋅⋅HNa, X2CH2⋅⋅⋅HNa (X = F, Cl, and Br) Binary and Ternary Complexes: A DFT and DFT-D3 Approach. Russ. J. Phys. Chem. 97, 3068–3080 (2023). https://doi.org/10.1134/S0036024423130277
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423130277