Abstract
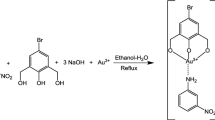
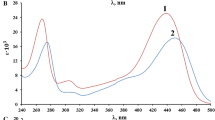
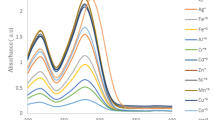
Kinetics of the reaction of ninhydrin (Nin) with baclofen (Bac) has been studied spectrophotometrically in an aqueous acidic medium under pseudo order conditions over 30–50°C range, 0.1–0.4 × 10–4 M of Bac, 0.5–5.0 × 10–2 M Nin, and ionic strength 0.3–0.9 M. The thermodynamics activation parameters involving ∆H* and ∆S* have been calculated. The UV-visible spectroscopic measurements were carried out to confirm the coupling between Nin and Bac. The reaction is first order with respect to [Nin] and [Bac], decreases as pH increases in the range (3.90–5.06). The experimental rate law is consistent with a mechanism in which the protonated and deprotonated form of Nin are involved in the rate-determining step and the deprotonated species is the more reactive one. The product of the reaction was examined spectroscopically using 1H-NMR and IR spectra in addition to an ultra-performance liquid chromatograph (UPLC). Density functional theory (DFT) was performed to search the geometries of the final product result from the reaction between Nin and Bac. Also, enthalpy of the reaction was calculated theoretically with DFT. Interaction region indicator (IRI) calculations is used to reveal chemical bonding and weak interaction in the coupled compound of Bac–Nin.












Similar content being viewed by others
REFERENCES
D. Kumar and M. A. Rub, J. Mol. Liq. 274, 639 (2019).
F. A. Siddiqui, N. Sher, N. Shafi, H. Shamshad, and A. Zubair, J. Anal. Sci. Technol. 4, 1 (2013).
B. H. Stuart, Forensic Analytical Techniques (Wiley, West Sussex, 2013).
A. B. Khan, A. Bhattarai, Z. H. Jaffari, B. Saha, and D. Kumar, Colloid Polym. Sci. 299, 1285 (2021).
D. Kumar and M. A. Rub, J. Surfact. Deterg. 22, 1299 (2019).
D. Kumar, M. A. Rub, and A. M. Asiri, R. Soc. Open Sci. 7, 200775 (2020).
B. Fry, J. F. Carter, K. Yamada, N. Yoshida, and D. Juchelka, Rapid Commun. Mass Spectrom. 32, 992 (2018).
K. A. Omar and R. Sadeghi, J. Mol. Liq. 303, 112644 (2020).
W. Meier-Augenstein, Stable Isotope Forensics: Methods and Forensic Applications of Stable Isotope Analysis (Wiley, New York, 2017).
N. G. Bowery, D. R. Hill, A. Hudson, A. Doble, D. N. Middlemiss, J. Shaw, and M. Turnbull, Nature (London, U.K.) 283, 92 (1980).
R. Gerkin, S. C. Curry, M. V. Vance, P. W. Sankowski, and R. D. Meinhart, Ann. Emerg. Med. 15, 843 (1986).
N. G. Bowery, D. R. Hill, and A. Hudson, Brit. J. Pharmacol. 78, 191 (1983).
S. J. Hosseinimehr, F. Pourmorad, E. Moshtaghi, and M. Amini, Asian J. Chem. 22, 522 (2010).
D. E. Hansel, C. R. Hansel, M. K. Shindle, E. M. Reinhardt, L. Madden, E. B. Levey, and A. H. Hoon, Jr., Pediat. Neurol. 29, 203 (2003).
M. M. Hefnawy and H. Y. Aboul-Enein, Talanta 61, 667 (2003).
R. Goda, N. Murayama, Y. Fujimaki, and K. Sudo, J. Chromatogr., B 801, 257 (2004).
S. Y. Chang, N. Y. Zheng, and C. S. Chen, Int. J. Appl. Sci. Eng. 2, 277 (2004).
H. E. Abdellatef and H. M. Khalil, J. Pharm. Biomed. Anal. 31, 209 (2003).
C. Zhang, R. Zhang, S. Zhang, M. Xu, and S. Zhang, Trials 15, 1 (2014).
R. R. Young, New England J. Med. 304, 96 (1981).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, et al., Gaussian 09, Revision C.01 (Gaussian Inc., Wallingford, 2009).
T. Lu and F. Chen, J. Comput. Chem. 33, 580 (2012).
W. Humphrey, A. Dalke, and K. Schulten, J. Mol. Graph. 14, 33 (1996).
T. Jézéquel, V. Joubert, P. Giraudeau, G. S. Remaud, and S. Akoka, Magn. Reson. Chem. 55, 77 (2017).
K. Bayle, M. Grand, A. Chaintreau, R. J. Robins, W. Fieber, H. Sommer, and G. S. Remaud, Anal. Chem. 87, 7550 (2015).
Handbook of Proton-NMR and Data (Asahi Res. Centre, Academic, Japan, 1985), Vol. 3.
R. H. Khan, J. Chem. Res. 6, 290 (2000).
T. M. Ansari, A. Raza, and A. U. Rehman, Anal. Sci. 21, 1133 (2005).
D. S. Hage, Reference Module in Chemistry, Molecular Sciences, and Chemical Engineering (Elsevier, Amsterdam, 2013).
V. Khlebnikov, J. Wijnen, W. J. van der Kemp, and D. W. Klomp, Ann. Rep. NMR Spectrosc. 87, 319 (2016).
E. Pandey and S. K. Upadhyay, J. Dispers. Sci. Technol. 27, 213 (2006).
M. Bano and I. A. Khan, Ind. J. Chem. 42, 1132 (2003).
T. Lu and Q. Chen, Chem. Methods 1, 231 (2021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim, T.A., Khaled, E.S., Mohamed, R.A. et al. Coupling Interaction of Baclofen with Ninhydrin in Aqueous Acidic Medium: Kinetics and Computational Studies. Russ. J. Phys. Chem. 97, 2985–2994 (2023). https://doi.org/10.1134/S0036024423130228
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423130228