Abstract
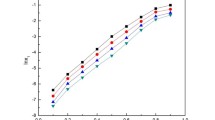
Cefpodoxime proxetil (CP) solubility in binary solvent mixtures (1,4-dioxane + water and acetonitrile + water) was tested using the gravimetric method at temperature between 298.15 to 318.15 K and under normal atmospheric pressure. The experimental mole fraction solubility of CP was increased with increasing temperature as well as with the proportion of co-solvent in all systems. Then Apelblat, Buchowski-Ksiazczak, Van’t Hoff, \(CNIBS{\text{/}}R{-} K\) and the modified Jouyban-Acree models were used for the prediction of the theoretical mole fraction solubility of CP. The experimental and theoretical mole fraction solubility were found to be good in this arrangement. Additionally, thermodynamics modeling of CP was carried out by using Van’t Hoff’s equation. The data on the solubility and thermodynamics would be useful in the production, isolation, separation, and crystallization of CP.



Similar content being viewed by others
REFERENCES
S. Ahmed, H. M. Abdel-Wadood, and N. A. Mohamed, J. Chromatogr., Ser. B 934, 34 (2013).
G. Patel and S. Rajput, Acta Chromatogr. 23, 215 (2011).
V. K. Kakumanu, V. K. Arora, and A. K. Bansal, J. Chromatogr., Ser. B 835, 16 (2006).
G. Agrawal and S. Bhargava, Curr. Drug Deliv. 5, 1 (2008).
A. O. Ebenezer, C.Chioma, and I. M. Omoegbe, J. Pharm. Pharmacol. 5, 1 (2017).
A. Bajaj, M. R. P. Rao, I. Khole, and G. Munjapara, Drug Develop. Ind. Pharm. 39, 635 (2013).
M. Nappinnai and S. Sivaneswari, J. Pharm. Res. 7, 304 (2013).
A. Mujtaba, M. Ali, and K. Kohli, Chem. Eng. Res. Des. 92, 156 (2014).
R. Bhola, H. Vaghani, K. Bhatt, J. Parikh, and R. Ghumara, Chem. Africa 5, 899 (2022).
R. Bhola, H. Modi, C. Patel, H. Vaghani, K. Bhatt, and R. Ghumara, J. Ind. Chem. Soc. 99, 100427 (2022).
M. Li, Y. Liu, M. Li, Z. Shang, M. Liu, and D. Han, Fluid Phase Equilib. 539, 113027 (2021).
J. Luo, Y. Wang, C. Shi, F. Zhang, and Q. Yu, J. Chem. Thermodyn. 168, 106748 (2022).
S. Yu, W. Xing, F. Xue, Y. Cheng, and B. Li, J. Chem. Thermodyn. 152, 106259 (2021).
L. Wang, D. Li, L. Wang, H. Hao, and L. Zhou, J. Chem. Eng. Data 66, 4593 (2021).
H. Wei, N. Gao, and L. Dang, Trans. Tianjin Univ. 27, 460 (2021).
A. Apelblat and E. Manzurola, J. Chem. Thermodyn. 31, 85 (1999).
R. Ghumara, H. Modi, A. Prajapati, C. Patel, and P. Parsaniya, Russ. J. Phys. Chem. A 95, 21 (2021).
H. Buchowski, A. Ksiazczak, and S. Pietrzyk, J. Phys. Chem. 84, 975 (1980).
M. Barzegar-Jalali, P. Jafari, and A. Jouyban, Phys. Chem. Liq. 250, 1 (2022).
R. Bhola, R. Ghumara, C. Patel, K. Bhatt, S. Patel, J. Parikh, A. Desai, and H. Vaghani, J. Chem. Eng. Data 68, 744 (2023).
Y. Wu, H. Ma, and Y. Han, J. Chem. Thermodyn. 161, 106555 (2021).
J. Chen, A. Farajtabar, A. Jouyban, W. E. Acree, P. Zhu, and H. Zhao, J. Chem. Eng. Data 66, 3531 (2021).
S. Yu, Y. Cheng, W. Feng, W. Xing, H. Li, and F. Xue, J. Mol. Liq. 339, 116750 (2021).
H. Rezaei, E. Rahimpour, H. Zhao, F. Martinez, and A. Jouyban, J. Mol. Liq. 336, 116519 (2021).
I. P. Osorio, F. Martínez, M. Á. Peña, A. Jouyban, and W. E. Acree, Phys. Chem. Liq. 59, 890 (2021).
W. Xu, Y. Ma, and B. Feng, J. Chem. Eng. Data 67, 825 (2022).
Y. Wu, H. Ma, nd Y. Han, J. Chem. Thermodyn. 161, 106555 (2021).
A. Ahad, F. Shakeel, M. Raish, A. Ahmad, Y. A. bin Jardan, F. I. Al-Jenoobi, and A. M. Al-Mohizea, J. Therm. Anal. Calorim. 147, 3117 (2022).
S. Alvani-Alamdari, H. Rezaei, E. Rahimpour, S. Hemmati, F. Martinez, M. Barzegar-Jalali, and A. Jouyban, Phys. Chem. Liq. 59, 12 (2021).
M. A. Kalam, A. Alshamsan, M. Alkholief, I. A. Alsarra, R. Ali, N. Haq, M. K. Anwer, and F. Shakeel, ACS Omega 5, 1708 (2020).
K. Kodide, P. Asadi, and J. Thati, J. Chem. Eng. Data 64, 5196 (2019).
A. Romdhani, F. Martínez, Á. Peña, E. Rahimpour, A. Jouyban, and W. E. Acree, Phys. Chem. Liq. 60, 203 (2022).
H. Rezaei, E. Rahimpour, H. Zhao, F. Martinez, M. Barzegar-Jalali, and A. Jouyban, J. Mol. Liq. 347, 118352 (2022).
K. Vakili, H. Rezaei, K. Poturcu, A. Jouyban, J. Hanaee, F. Martinez, and E. Rahimpour, J. Mol. Liq. 344, 117915 (2021).
T. Sayad, K. Poturcu, M. Moradi, E. Rahimpour, H. Zhao, and A. Jouyban, J. Mol. Liq. 342, 117537 (2021).
D. R. Delgado, O. Bahamón-Hernandez, N. E. Cerquera, C. P. Ortiz, F. Martínez, E. Rahimpour, A. Jouyban, and W. E. Acree, J. Mol. Liq. 322, 114979 (2021).
P. Cysewski, T. Jeliński, D. Procek, and A. Dratwa, Fluid Phase Equilib. 529, 112883 (2021).
M. Barzegar-Jalali, E. Mazaher Haji Agha, K. Adibkia, S. Hemmati, F. Martinez, and A. Jouyban, Phys. Chem. Liq. 59, 690 (2021).
R. Bhola, R. Ghumara, K. Bhatt, and H. Vaghani, Biointerface Res. Appl. Chem. 12, 4374 (2021).
ACKNOWLEDGMENTS
The authors are thankful to the Principal, MUIS, Ganpat University for providing basic laboratory facility and also grateful to Mann Pharmaceutical for the providing CP for the analysis, and also thankful to Prof. Sagar Patel and Prof. Kinjal Joshi for the proof reading of the manuscript.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Bhola, R., Ghumara, R., Patel, C. et al. Antibiotic Drug Solubility and Thermodynamics Profile in Binary (1,4-Dioxane, Acetonitrile, and Water) Solvents at Different Temperatures (T = 298.15–318.15 K). Russ. J. Phys. Chem. 97, 2915–2924 (2023). https://doi.org/10.1134/S0036024423130186
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423130186