Abstract
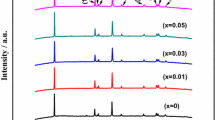
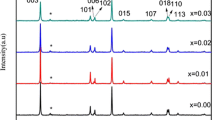
LiNi0.5Co0.2Mn0.3O2 (NCM523) cathode materials are susceptible to irreversible phase changes and surface side reactions between electrode materials and electrolyte during charging and discharging, which represent a severe danger to the safety and electrochemical performance of batteries. Li\(_{{1.06-x}}\)NaxNi0.5Co0.2Mn0.3O\(_{{2-y}}\)Cly cathode materials co-doped with Na+ and Cl– were prepared by a simple co-precipitation method. The effects of crystal structure, morphology and electrochemical properties were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), electrochemical impedance spectroscopy (EIS) and charge-discharge performance tests. The results show that the doping of Na+ and Cl– reduces the mixing of cations and expands the diffusion channels of Li+. Currently, the doped 1%-NCM has a high charge-discharge capacity and the best cycling performance (187.56 mA h g–1 at 1 C with the highest capacity retention at 76.29% after the first cycle). After 50 cycles, the specific capacity retention was 138.33 mA h g–1, which increased the capacity retention by 18.58% compared with the unmodified material, indicating that the co-doping of anions and cations improved the Li+ de-embedding ability as well as the specific capacity.





Similar content being viewed by others
REFERENCES
S. Yang, X. Wang, X. Yang, et al., Electrochim. Acta 66, 88 (2012).
M. Noh and J. Cho, J. Electrochem. Soc. 160, A105 (2013).
W. Liu, P. Oh, X. Liu, et al., Angew. Chem. Int. Ed. 54, 4440 (2015).
J. M. Tarascon and M. Armand, Nature (London, U.K.) 414, 359 (2001).
R. A. Leising, M. J. Palazzo, E. S. Takeuchi, et al., J. Electrochem. Soc. 148, A838 (2001).
H. Zhu, C. Miao, R. Guo, et al., Int. J. Electrochem. Sci. 16, 210331 (2021).
R. A. Rodríguez, M. G. Montiel, N. D. S. Mohallem, et al., Solid State Ionics 369, 115707 (2021).
S. Cui, Y. Wei, T. Liu, et al., Adv. Energy Mater. 6, 1501309 (2016).
M. Hagen, W. J. Cao, A. Shellikeri, et al., J. Power Sources 379, 212 (2018).
J. Xiong, T. Zheng, Y. J. Cheng, et al., ACS Appl. Mater. Interfaces 13, 18648 (2021).
J. B. Goodenough and Y. Kim, Chem. Mater. 22, 587 (2010).
Z. X. Huang, Z. Xu, Z. G. Ji, et al., Chin. J. Rare Met. 44, 860 (2020).
L. Feng, Y. Liu, W. Qin, et al., Electrochim. Acta 391, 138904 (2021).
W. Hua, J. Zhang, Z. Zheng, et al., Dalton Trans. 43, 14824 (2014).
D. Aurbach, O. Srur-Lavi, C. Ghanty, et al., J. Electrochem. Soc. 162, A1014 (2015).
Y. Zhang, Z. B. Wang, J. Lei, et al., Ceram. Int. 41, 9069 (2015).
Y. H. Chen, J. Zhang, Y. R. Ren, et al., J. Phys. Chem. C 125, 19600 (2021).
Y. Q. Sun, W. Fu, Y. X. Hu, et al., Tungsten 3, 245 (2021).
L. Xia, K. Qiu, Y. Gao, et al., J. Mater. Sci. 50, 2914 (2015).
X. Li, H. Xie, H. Jin, et al., Nano 16, 2150159 (2021).
J. H. Song, J. Bae, K. Lee, et al., J. Ind. Eng. Chem. 68, 124 (2018).
G. Shang, Y. Tang, Y. Lai, et al., J. Power Sources 423, 246 (2019).
X. Jia, M. Yan, Z. Zhou, et al., Electrochim. Acta 254, 50 (2017).
Y. Jiang, Y. Bi, M. Liu, et al., Electrochim. Acta 268, 41 (2018).
T. Ohzuku and A. Ueda, J. Electrochem. Soc. 141, 2972 (1994).
T. Ohzuku, M. Nagayama, K. Tsuji, et al., J. Mater. Chem. 21, 10179 (2011).
A. van Bommel, L. J. Krause, and J. R. Dahn, J. Electrochem. Soc. 158, A731 (2011).
Y. Ren, A. R. Armstrong, F. Jiao, et al., J. Am. Chem. Soc. 132, 996 (2010).
R. Zhao, Z. Yang, J. Liang, et al., J. Alloys Compd. 689, 318 (2016).
Z. Chen, X. Gong, H. Zhu, et al., Front. Chem. 6, 643 (2019).
S. Singh, A. K. Raj, R. Sen, et al., ACS Appl. Mater. Interfaces 9, 26885 (2017).
W. K. Kim, D. W. Han, W. H. Ryu, et al., J. Alloys Compd. 592, 48 (2014).
D. Wang, X. Li, Z. Wang, et al., J. Alloys Compd. 647, 612 (2015).
Y. Xu, Y. Liu, Z. Lu, et al., Appl. Surf. Sci. 361, 150 (2016).
L. Xia, K. Qiu, Y. Gao, et al., J. Mater. Sci. 50, 2914 (2015).
Funding
The authors gratefully acknowledge supports by the Natural Science Foundation of Liaoning Province (no. 2020-BS-154).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, X., Dai, S., Li, H. et al. Preparation and Electrochemical Investigation of Na and Cl Co-Doped LiNi0.5Co0.2Mn0.3O2 Cathode Materials. Russ. J. Phys. Chem. 97, 3168–3173 (2023). https://doi.org/10.1134/S003602442313023X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442313023X