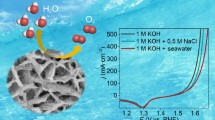
Abstract
For the heterogeneous alloy catalysts of water electrolysis, it has been reported that conductivity can be improved through structural modifications by introducing other elements like chalcogens. Transition metal sulfides can induce numerous lattice defects due to their unique interface formation, thereby promoting abundant active sites and facilitating electron/ion movement. In this study, we report the enhanced electrochemical activity of NiFeS formed on nickel foam (NiFeS@NF) for oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) during the water electrolysis, especially, the seawater electrolysis. NiFeS@NF synthesized through a one-step electrochemical deposition had an amorphous-like highly porous structure with the aggregates of spherical nanoparticles attached to nickel foam. Compared to NiFe@NF, NiFeS@NF catalysts demonstrated a reduced overpotential by ~32 mV and ~96 mV for OER and HER, respectively, at 100 mA cm−2 and secured electrochemical stability over 24 h. Moreover, bifunctional seawater electrolysis using NiFeS@NF as both electrodes demonstrated the reduced overpotential by ~80 mV with durability over time. This facile synthesis method for anion doping and the enhanced and selective electrolysis of seawater without producing Cl2 gas holds promise for the creation of high-performance electrocatalysts applicable in a wide range of hydrogen energy-related fields.
Graphical Abstract








Similar content being viewed by others
References
Lal, R.: Carbon sequestration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 815–830 (2008)
Maggio, G., Nicita, A., Squadrito, G.: How the hydrogen production from RES could change energy and fuel markets: a review of recent literature. Int. J. Hydrog. Energy 44, 11371–11384 (2019)
Pudukudy, M., Yaakob, Z., Mohammad, M., Narayanan, B., Sopian, K.: Renewable hydrogen economy in Asia–opportunities and challenges: an overview. Renew. Sust. Energ. Rev. 30, 743–757 (2014)
Alobaid, A., Wang, C., Adomaitis, R.A.: Mechanism and kinetics of HER and OER on NiFe LDH films in an alkaline electrolyte. J. Electrochem. Soc. 165, J3395–J3404 (2018)
Tahir, M., Pan, L., Idrees, F., Zhang, X., Wang, L., Zou, J.J., Wang, Z.L.: Electrocatalytic oxygen evolution reaction for energy conversion and storage: a comprehensive review. Nano Energy 37, 136–157 (2017)
Wu, Z.P., Lu, X.F., Zang, S.Q., Lou, X.W.: Non-noble‐metal‐based electrocatalysts toward the oxygen evolution reaction. Adv. Funct. Mater. 30, 1910274 (2020)
Mohammed-Ibrahim, J., Moussab, H.: Recent advances on hydrogen production through seawater electrolysis. Mater. Sci. Technol. 3, 780–807 (2020)
Wang, C., Shang, H., Jin, L., Xu, H., Du, Y.: Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale 13, 7897–7912 (2021)
D’Amore-Domenech, R., Leo, T.J.: Sustainable hydrogen production from offshore marine renewable farms: techno-energetic insight on seawater electrolysis technologies. ACS Sustain. Chem. Eng 7, 8006–8022 (2019)
Dresp, S., Dionigi, F., Klingenhof, M., Strasser, P.: Direct electrolytic splitting of seawater: opportunities and challenges. ACS Energy Lett. 4, 933–942 (2019)
Liu, W., Jiang, K., Hu, Y., Li, Q., Deng, Y., Bao, J., Lei, Y.: Zr-doped CoFe-layered double hydroxides for highly efficient seawater electrolysis. J. Colloid Interface Sci. 604, 767–775 (2021)
Jadhav, A.R., Kumar, A., Lee, J., Yang, T., Na, S., Lee, J., Lee, H.: Stable complete seawater electrolysis by using interfacial chloride ion blocking layer on catalyst surface. J. Mater. Chem. A 8, 24501–24514 (2020)
Chen, J., Zhang, L., Li, J., He, X., Zheng, Y., Sun, S., Fang, X., Zheng, D., Luo, Y., Wang, Y., Zhang, J., Xie, L., Cai, Z., Sun, Y., Alshehri, A.A., Kong, Q., Tang, C., Sun, X.: High-efficiency overall alkaline seawater splitting using a nickel–iron sulfide nanosheet array as a bifunctional electrocatalyst. J. Mater. Chem. A 11, 1116–1122 (2023)
Tu, Q., Liu, W., Jiang, M., Wang, W., Kang, Q., Wang, P., Zhou, W., Zhou, F.: Preferential adsorption of hydroxide ions onto partially crystalline NiFe-layered double hydroxides leads to efficient and selective OER in alkaline seawater. ACS Appl. Energy Mater. 4, 4630–4637 (2021)
Zhang, B., Liu, S., Zhang, S., Cao, Y., Wang, H., Han, C.: High corrosion resistance of NiFe-layered double hydroxide catalyst for stable seawater electrolysis promoted by phosphate intercalation. Small 18, 2203852 (2022)
Cui, B., Hu, Z., Liu, C., Liu, S., Chen, F., Hu, S., Hu, W.: Heterogeneous lamellar-edged Fe-Ni(OH) 2/Ni3S2 nanoarray for efficient and stable seawater oxidation. Nano Res. 14, 1149–1155 (2021)
Shang, X., Qin, J.F., Lin, J.H., Dong, B., Chi, J.Q., Liu, Z.Z., Liu, C.G.: Tuning the morphology and Fe/Ni ratio of a bimetallic Fe-Ni-S film supported on nickel foam for optimized electrolytic water splitting. J. Colloid Interface Sci. 523, 121–132 (2018)
Fan, H., Ma, Y., Chen, W., Tang, Y., Li, L., Wang, J.: Facile one-step electrodeposition of two-dimensional nickel-iron bimetallic sulfides for efficient electrocatalytic oxygen evolution. J. Alloys Compd. 894, 162533 (2022)
Pan, Z., Yaseen, M., Shen, P.K., Zhan, Y.: Designing highly efficient 3D porous Ni-Fe sulfide nanosheets based catalyst for the overall water splitting through component regulation. J. Colloid Interface Sci. 616, 422–432 (2022)
Sang, Y., Ding, G., Guo, Z., Xue, Y., Li, G., Zhang, R.: Facile synthesis of amorphous bimetallic hydroxide on Fe-doped Ni3S2 as an active electrocatalyst for oxygen evolution reaction. J. Alloys Compd. 919, 165855 (2022)
Wang, A.L., Xu, H., Li, G.R.: NiCoFe layered triple hydroxides with porous structures as high-performance electrocatalysts for overall water splitting. ACS Energy Lett. 1, 445–453 (2016)
Yu, L., Wu, L., McElhenny, B., Song, S., Luo, D., Zhang, F., Ren, Z.: Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy) hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 13, 3439–3446 (2020)
Dong, B., Zhao, X., Han, G.Q., Li, X., Shang, X., Liu, Y.R., Liu, C.G.: Two-step synthesis of binary Ni–Fe sulfides supported on nickel foam as highly efficient electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 4, 13499–13508 (2016)
Acknowledgements
This work was supported by Kyonggi University’s Graduate Research Assistantship 2022 and Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE) (P0020614, HRD Program for Industrial Innovation).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, D., Ryu, S. Efficient and Selective Oxygen Evolution Reaction in Seawater Electrolysis with Electrochemically Synthesized Amorphous-like NiFeS. Electron. Mater. Lett. 20, 173–182 (2024). https://doi.org/10.1007/s13391-023-00476-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-023-00476-7