Abstract
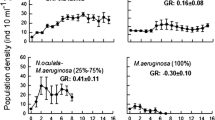
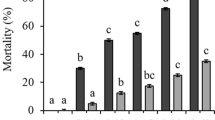
In order to assess the impact of cyanobacteria on mollusks under experimental conditions, the interaction of toxic and nontoxic strains of the cyanobacterium Microcystis aeruginosa (Kützing) Kützing and bivalve mollusks Unio pictorum (Linnaeus, 1758) has been studied. Cyanobacteria have a negative effect on bivalve mollusks: the 40% death of mollusks and deterioration of their adaptive capacity are recorded when cocultivated with M. aeruginosa at a high cell concentration. At the same time, there is no difference in the mortality of mollusks incubated with toxic and nontoxic cyanobacteria. A decrease in the content of microcystin-LR in the presence of bivalves is revealed. No statistically significant increase in the number of cyanobacteria in the water is noted after transit passage through the digestive system of bivalves.


Similar content being viewed by others
Notes
Cyanobacterial toxins: microcystins. Background document for development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments. Geneva: World Health Organization 2020 (WHO/HEP/ECH/WSH/2020.6). License: CC BY-NCSA 3.0 IGO.
REFERENCES
Alimov, A.F., Funktsional’naya ekologiya presnovodnykh dvustvorchatykh mollyuskov (Functional Ecology of Freshwater Bivalves), Leningrad: Nauka, 1981.
Bakhmet, I.N., Cardiac activity and oxygen consumption of blue mussels (Mytilus edulis) from the White Seain relation to body mass, ambient temperature and food availability, Polar Biol., 2017, vol. 40, p. 1959. https://doi.org/10.1007/s00300-017-2111-6
Berezina, N.A., Tolerance of freshwater invertebrates to changes in water salinity, Russ. J. Ecol., 2003, vol. 34, no. 4, p. 261. https://doi.org/10.1023/A:1024597832095
Berezina, N.A., Maximov, A.A., Umnova, L.P., et al., Excretion by benthic invertebrates as important source of phosphorus in oligotrophic ecosystem (Lake Krivoe, northern Russia), J. Sib. Fed. Univ., Biol., 2017, vol. 10, no. 4, p. 485. https://doi.org/10.17516/1997-1389-0046
Berezina, N.A., Verbitsky, V.B., Sharov, A.N., and Chernova, E., Biomarkers in bivalve mollusks and amphipods for assessment of effects linked to cyanobacteria and elodea: Mesocosm study, Ecotoxicol. Environ. Saf., 2020, vol. 203, p. 110994. https://doi.org/10.1016/j.ecoenv.2020.110994
Berezina, N.A., Tiunov, A.V., Tsurikov, S.M., et al., Cyanobacteria as a food source for invertebrates: Results of a model experiment, Russ. J. Ecol., 2021, vol. 52, pp. 247–252. https://doi.org/10.1134/S1067413621030036
Boegehold, A.G., Johnson, N.S., and Kashian, D.R., Dreissenid (quagga and zebra mussel) veligers are adversely affected by bloom forming cyanobacteria, Ecotoxicol. Environ. Saf., 2019, vol. 182, p. 109426. https://doi.org/10.1016/j.ecoenv.2019.109426
Bownik, A., Effects of cyanobacterial toxins, microcystins on freshwater invertebrates, Pol. J. Nat. Sci., 2013, vol. 28, no. 2, p. 185.
Burnett, N.P., Seabra, R., De Pirro, M., and Davis, S.W., An improved noninvasive method for measuring heartbeat of intertidal animals, Limnol. Oceanogr. Methods, 2013, vol. 11, p. 91. https://doi.org/10.4319/lom.2013.11.91
Davis, T.W. and Gobler, C.J., Grazing by mesozooplankton and microzooplankton on toxic and non-toxic strains of Microcystis in the Transquaking River, a tributary of Chesapeake Bay, J. Plankton Res., 2010, vol. 33, no. 3, p. 415. https://doi.org/10.1093/plankt/fbq109
Depledge, M.H., Aagaard, A., and Gyorkos, P., Assessment of trace metal toxicity using molecular, physiological and behavioral biomarkers, Mar. Pollut. Bull., 1995, vol. 31, p. 19. https://doi.org/10.1016/0025-326X(95)00006-9
Dionisio Pires, L.M., Bontes, B.M., Van Donk, E., and Ibelings, B.W., Grazing on colonial and filamentous, toxic and non-toxic cyanobacteria by the zebra mussel Dreissena polymorpha, J. Plankton Res., 2005, vol. 27, no. 4, p. 331. https://doi.org/10.1093/plankt/fbi008
Gagné, F., Gélinas, M., Fortier, M., and Fournier, M., The effects of cyanobacterial blooms on the immune system of Elliptio complanata in urban and agricultural areas in the Yamaska River watershed, Invertebr. Survival J., 2018, vol. 15, p. 39.
Ger, K.A., Arneson, P., Goldman, C.R., and The, S.J., Species specific differences in the ingestion of Microcystis cells by the calanoid copepods Eurytemora affinis and Pseudodiaptomus forbesi, J. Plankton Res., 2010a, vol. 32, no. 10, p. 1479. https://doi.org/10.1093/plankt/fbq071
Ger, K.A., Teh, S.J., Baxa, D.V., et al., The effects of dietary Microcystis aeruginosa and microcystin on the copepods of the upper San Francisco Estuary, Freshwater Biol., 2010b, vol. 55, no. 7, p. 1548. https://doi.org/10.1111/j.1365-2427.2009.02367.x
Gibble, C.M., Peacock, M.B., and Kudela, R.M., Evidence of freshwater algal toxins in marine shellfish: Implications for human and aquatic health, Harmful Algae, 2016, vol. 59, p. 59. https://doi.org/10.1016/j.hal.2016.09.007
Jeffrey, S.W. and Humprhråy, G.E., New spectrophotometric equations for determining chlorophylls a, b, c 1 and c 2 in higher plants, algae and natural phytoplankton, Biochim. Physiol., 1975, vol. 167, no. 2, p. 191. https://doi.org/10.1016/s0015-3796(17)30778-3
Kholodkevich, S.V., Sharov, A.N., Chuiko, G.M., et al., Quality assessment of freshwater ecosystems by the functional state of bivalved mollusks, Water Resour., 2019, vol. 46, pp. 249–257. https://doi.org/10.1134/S0097807819020064
Kholodkevich, S.V., Chuiko, G.M., Sharov, A.N., et al., Indicators of cardiac activity and oxidative stress in the mollusk Anodonta cygnea under short-term salt test load as biomarkers for assessing the state of the organism and the quality of the environment, Inland Water Biol., 2021, vol. 14, no. 6, p. 739. https://doi.org/10.1134/S1995082921060067
Klishko, O., Lopes-Lima, M., Froufe, E., et al., Taxonomic reassessment of the freshwater mussel genus Unio (Bivalvia: Unionidae) in Russia and Ukraine based on morphological and molecular data, Zootaxa, 2017, vol. 4286, no. 1, p. 93. https://doi.org/10.11646/zootaxa.4286.1.4
Kolmakov, V.I., Role of Microcystis aeruginosa passing through the digestive tracts of filter-feeding animals in eutrophic water reservoirs (review), Contemp. Probl. Ecol., 2014, vol. 7, no. 4, p. 455. https://doi.org/10.1134/S1995425514040052
Kolmakov, V.I. and Gladyshev, M.I., Conceptual diversicology—A new section of theoretical ecology, Gidrobiol. Zh., 2003, vol. 39, no. 4, p. 111.
Komendantov, A.Yu., Khlebovich, V.V., and Aladin, N.V., Features of osmotic and ionic regulation of bivalves depending on environmental factors, Ekologiya, 1985, no. 5, p. 35.
Kurbatova, S.A., Berezina, N.A., Sharov, A.N., et al., Interactions of cyanobacteria and aquatic organisms: Can crustaceans facilitate cyanobacteria bloom?, Russ. J. Ecol., 2022, vol. 53, no. 6, p. 565. https://doi.org/10.1134/S1067413622060078
Medvedeva, N., Zaytseva, T., and Kuzikova, I., Cellular responses and bioremoval of nonylphenol by the bloom-forming cyanobacterium Planktothrix agardhii 1113, J. Mar. Syst., 2017, vol. 171, p. 120. https://doi.org/10.1016/j.jmarsys.2017.01.009
Merel, S.D., Walker, R., Chicana, Sh., Snyder, et al., State of knowledge and concerns on cyanobacterial blooms and cyanotoxins, Environ. Int., 2013, vol. 59, p. 303. https://doi.org/10.1016/j.envint.2013.06.013
Miller, M.A., Kudela, R.M., Mekebri, A., et al., Evidence for a novel marine harmful algal bloom: Cyanotoxin (microcystin) transfer from land to sea otters, PLoS One, 2010, vol. 5, no. 9, e12576. https://doi.org/10.1371/journal.pone.0012576
Mohamed, Z.A., Bakr, A.A., and Ghramh, H.A., Grazing of the copepod Cyclops vicinus on toxic Microcystis aeruginosa: potential for controlling cyanobacterial blooms and transfer of toxins, Oceanol. Hydrobiol. Stud., 2018, vol. 47, no. 3, p. 296. https://doi.org/10.1515/ohs-2018-0028
Ostroumov, S.A., Gidrobionty v samoochishchenii vod i biogennoi migratsii elementov (Hydrobionts in Self-purification of Water and Biogenic Migration of Elements), Moscow: MAKS, 2008.
Paerl, H.W., Controlling cyanobacterial harmful blooms in freshwater ecosystems, Microb. Biotechnol., 2017, vol. 10, no. 5, p. 1106. https://doi.org/10.1111/1751-7915.12725
Paldavičienė, A., Zaiko, A., Mazur-Marzec, H., and Razinkovas-Baziukas, A., Bioaccumulation of microcystins in invasive bivalves: A case study from the boreal lagoon ecosystem, Oceanologia, 2015, vol. 57, no. 1, p. 93. https://doi.org/10.1016/j.oceano.2014.10.001
Rippka, R., Deruelles, J., Waterbury, J.B., et al., Genetic assignments, strain histories and properties of pure cultures of cyanobacteria, Microbiology, 1979, vol. 111, p. 1. https://doi.org/10.1099/00221287-111-1-1
Sipiä, V.O., Kankaanpää, H.T., Pflugmacher, S., et al., Bioaccumulation and detoxication of nodularin in tissues of flounder (Platichthys flesus), Mussels (Mytilus edulis, Dreissena polymorpha), and clams (Macoma balthica) from the Northern Baltic Sea, Ecotoxicol. Environ. Saf., 2002, vol. 53, no. 2, p. 305. https://doi.org/10.1006/eesa.2002.2222
Sitnikova, T., Kiyashko, S.I., Maximova, N., et al., Resource partitioning in endemic species of Baikal gastropods indicated by gut contents, stable isotopes and radular morphology, Hydrobiologia, 2012, vol. 682, p. 75. https://doi.org/10.1007/s10750-011-0685-5
Sutradhar, M., The current scenario and future aspects of Cyanotoxins: A Review Study, J. Mater. Environ. Sci., 2022, vol. 13, no. 07, p. 768.
Vanderploeg, H.A., Johengen, T.H., and Liebig, J.R., Feedback between zebra mussel selective feeding and algal composition affects mussel condition: Did the regime changer pay a price for its success?, Freshwater Biol., 2009, vol. 54, no. 1, p. 47. https://doi.org/10.1111/j.1365-2427.2008.02091.x
Verbitsky, V.B., Kurbatova, S.A., Berezina, N.A., et al., Responses of aquatic organisms to cyanobacteria and elodea in microcosms, Dokl. Biol. Sci., 2018, vol. 488, p. 136. https://doi.org/10.1134/S0012496619050028
Wood, R., Acute animal and human poisonings from cyanotoxin exposure—A review of the literature, Environ. Int., 2016, vol. 91, p. 276. https://doi.org/10.1016/j.envint.2016.02.026
Xing, Q., Zhang, L., Li, Y., et al., Development of novel cardiac indices andassessment of factors affecting cardiac activity in a bivalve mollusk Chlamys farreri, Front. Physiol., 2019, vol. 10, p. 293. https://doi.org/10.3389/fphys.2019.00293
Zurawell, R.W., Chen, H., Burke, J.M., and Prepas, E.E., Hepatotoxic cyanobacteria: A review of the biological importance of microcystins in freshwater environments, J. Toxicol. Environ. Health, Part B, 2005, vol. 8, no. 1, p. 1. https://doi.org/10.1080/10937400590889412
ACKNOWLEDGMENTS
We thank S.V. Kholodkevich (St. Petersburg Federal Research Center, Russian Academy of Sciences) for providing equipment for recording the heart rate in mollusks.
Funding
The work was carried out as part of the state task of the Ministry of Science and Higher Education of the Russian Federation, topics 122041100085-8, 122041100086-5, and 121051100099-5, as well as with financial support from the Government of the Tyumen oblast according to the project of the West Siberian Interregional Scientific and Educational Center, no. 89-DON (2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: HPLC, high performance liquid chromatography; Chl, chlorophyll; HR, heart rate.
Rights and permissions
About this article
Cite this article
Sharov, A.N., Zaytseva, T.B. & Medvedeva, N.G. Responses of Unio pictorum to the Presence of Toxic and Nontoxic Strains of Microcystis aeruginosa. Inland Water Biol 16, 1159–1165 (2023). https://doi.org/10.1134/S1995082923060214
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082923060214