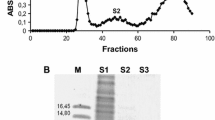
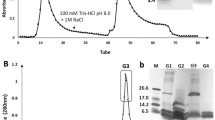
Abstract
Antifungal resistance poses a significant challenge to disease management, necessitating the development of novel drugs. Antimicrobial peptides offer potential solutions. This study focused on extraction and characterization of peptides from Adenanthera pavonina seeds with activity against Candida species, Mycobacterium tuberculosis, proteases, and α-amylases. Peptides were extracted in phosphate buffer and heated at 90°C for 10 min to create a peptide rich heated fraction (PRHF). After confirming antimicrobial activity and the presence of peptides, the PRHF underwent ion exchange chromatography, yielding retained and non-retained fractions. These fractions were evaluated for antimicrobial activity and cytotoxicity against murine macrophages. The least toxic and most active fraction underwent reversed-phase chromatography, resulting in ten fractions. These fractions were tested for peptides and antimicrobial activity. The most active fraction was rechromatographed on a reversed-phase column, resulting in two fractions that were assessed for antimicrobial activity. The most active fraction revealed a single band of approximately 6 kDa and was tested for inhibitory effects on proteases and α-amylases. Thermal stability experiments were conducted on the 6 kDa peptide at different temperatures followed by reassessment of antifungal activity and circular dichroism. The 6 kDa peptide inhibited yeasts, M. tuberculosis, human salivary and Tenebrio molitor larvae intestine α-amylases, and proteolytic activity from fungal extracts, and thus named ApPI. Remarkably, ApPI retained antifungal activity and conformation after heating and is primarily composed of α-helices. ApPI is a thermally stable serine protease/α-amylase inhibitor from A. pavonina seeds, offering promise as a foundational molecule for innovative therapeutic agents against fungal infections and tuberculosis.












Similar content being viewed by others
Data Availability
Data will be made available on request.
Abbreviations
- PRHF:
-
Peptide‒rich heated fraction
- IC50 :
-
Half maximal inhibitory concentration
- MTT:
-
3‒(4,5‒Dimethylthiazol‒2‒yl)‒2,5‒diphenyl‒tetrazolium bromide
- CFU:
-
Colony forming unit
- OD:
-
Optical density
- U:
-
Unit activity
References
Banerjee S, Denning D, Chakrabarti A (2021) One Health aspects & priority roadmap for fungal diseases: A mini-review. Indian J Med Res 153:311. https://doi.org/10.4103/ijmr.ijmr_768_21
Rodrigues ML, Nosanchuk JD (2020) Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl Trop Dis 14:e0007964. https://doi.org/10.1371/journal.pntd.0007964
McCarty TP, White CM, Pappas PG (2021) Candidemia and invasive candidiasis. Infect Dis Clin North Am 35:389–413. https://doi.org/10.1016/j.idc.2021.03.007
Dadar M, Tiwari R, Karthik K, Chakraborty S, Shahali Y, Dhama K (2018) Candida albicans – Biology, molecular characterization, pathogenicity, and advances in diagnosis and control – An update. Microb Pathog 117:128–138. https://doi.org/10.1016/j.micpath.2018.02.028
Giles C, Lamont-Friedrich SJ, Michl TD, Griesser HJ, Coad BR (2018) The importance of fungal pathogens and antifungal coatings in medical device infections. Biotechnol Adv 36:264–280. https://doi.org/10.1016/j.biotechadv.2017.11.010
Chakrabarti A, Sood P (2021) On the emergence, spread and resistance of Candida auris: Host, pathogen and environmental tipping points. J Med Microbiol 70(3):001318. https://doi.org/10.1099/jmm.0.001318
Spivak ES, Hanson KE (2018) Candida auris: An emerging fungal pathogen. J Clin Microbiol 56. https://doi.org/10.1128/jcm.01588-17
Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G (2020) Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog 16:e1008921. https://doi.org/10.1371/journal.ppat.1008921
Talapko J, Juzbašić M, Matijević T, Pustijanac E, Bekić S (2021) Candida albicans—the virulence factors and clinical manifestations of infection. J Fungi 7:79. https://doi.org/10.3390/jof7020079
von Lilienfeld-Toal M, Wagener J, Einsele H, Cornely OA, Kurzai O (2019) Invasive fungal infection. Dtsch Arztebl Int. https://doi.org/10.3238/arztebl.2019.0271
Costa-de-Oliveira S, Rodrigues AG (2020) Candida albicans antifungal resistance and tolerance in bloodstream infections: The triad yeast-host-antifungal. Microorganisms 8:154. https://doi.org/10.3390/microorganisms8020154
WHO fungal priority pathogens list to guide research, development and public health action. (2022) Geneva: World Health Organization. License: CC BY-NC-SA 3.0 IGO
Zink A, Haas CJ, Reischl U, Szeimies U, Nerlich AG (2021) Molecular analysis of skeletal tuberculosis in an ancient Egyptian population. J Med Microbiol 50(4):355–366. https://doi.org/10.1099/0022-1317-50-4-355
Linh NN, Viney K, Gegia M, Falzon D, Glaziou P, Floyd K, Timimi H, Ismail N, Zignol M, Kasaeva T, Mirzayev F (2021) World Health Organization treatment outcome definitions for tuberculosis: 2021 update. Eur Respir J 58:2100804. https://doi.org/10.1183/13993003.00804-2021
Khawbung JL, Nath D, Chakraborty S (2021) Drug resistant tuberculosis: A review. Comp Immunol Microbiol Infect Dis 74:101574. https://doi.org/10.1016/j.cimid.2020.101574
Amala SE, Hanson A, Wokem GN (2020) Candida coinfection with Mycobacterium tuberculosis in tuberculosis patients and antifungal susceptibility of the isolates. J Tuberc Res 08:53–65. https://doi.org/10.4236/jtr.2020.82006
Song W-M, Zhao J-Y, Zhang Q-Y, Lui S-Q, Zhu X-H, An Q-Q, Xu T-T, Liu J-Y, Tao N-N, Li LY, Y-F, Li H-C, (2021) COVID-19 and tuberculosis coinfection: An overview of case reports/case series and meta-analysis. Front Med (Lausanne) 8:657006. https://doi.org/10.3389/fmed.2021.657006
Daneshvar P, Hajikhani B, Sameni F, Noorisepehr N, Zare F, Bostanshirin N, Yazdani S, Goudarzi M, Sayyari S, Dadashi M (2023) COVID-19 and tuberculosis coinfection: An overview of case reports/case series and meta-analysis of prevalence studies. Heliyon 9:e13637. https://doi.org/10.1016/j.heliyon.2023.e13637
Moretta A, Scieuzo C, Petrone AM, Salvia R, Manniello MD, Franco A, Lucchetti D, Vassallo A, Vogel H, Sgambato A, Falabella P (2021) Antimicrobial peptides: A new hope in biomedical and pharmaceutical fields. Front Cell Infect Microbiol 11:668632. https://doi.org/10.3389/fcimb.2021.668632
Li S, Wang Y, Xue Z, Jia R, He C, Chen H (2021) The structure-mechanism relationship and mode of actions of antimicrobial peptides: A review. Trends Food Sci Technol 109:103–115. https://doi.org/10.1016/j.tifs.2021.01.005
Chung PY, Khanum R (2017) Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J Microbiol Immunol Infect 50:405–410. https://doi.org/10.1016/j.jmii.2016.12.005
Carrillo-Muñoz AJ, Finquelievich J, Tur-Tur C, Eraso S, Jauregizar N, Quindós G, Giusiano G (2014) Combination antifungal therapy: a strategy for the management of invasive fungal infections. Rev Esp Quimioter 27:141–158
Arranz-Trullén J, Lu L, Pulido D, Bhakta S, Boix E (2017) Host antimicrobial peptides: The promise of new treatment strategies against tuberculosis. Front Immunol 8:1499. https://doi.org/10.3389/fimmu.2017.01499
Lewies A, Du Plessis LH, Wentzel JF (2019) Antimicrobial peptides: The Achilles’ heel of antibiotic resistance? Probiotics Antimicrob Proteins 11:370–381. https://doi.org/10.1007/s12602-018-9465-0
Costa F, Teixeira C, Gomes P, Martins MCL (2019) Clinical application of AMPs. Em: Advances in Experimental Medicine and Biology. Springer Singapore, Singapore, p 281–298
Li X, Zuo S, Wang B, Zhang K, Wang Y (2022) Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules 27:2675. https://doi.org/10.3390/molecules27092675
Perez-Rodriguez A, Eraso E, Quindós G, Mateo E (2022) Antimicrobial peptides with anti–Candida activity. Int J Mol Sci 23:9264. https://doi.org/10.3390/ijms23169264
Mehta K, Sharma P, Mujawar S, Vyas A (2022) Role of antimicrobial peptides in treatment and prevention of Mycobacterium tuberculosis: A review. Int J Pept Res Ther 28:132. https://doi.org/10.1007/s10989-022-10435-9
Saini J, Kaur P, Malik N, Lakhawat SS, Sharma PK (2022) Antimicrobial peptides: A promising tool to combat multidrug resistance in SARS CoV-2 era. Microbiol Res 265:127206. https://doi.org/10.1016/j.micres.2022.127206
García-Olmedo F, Molina A, Alamillo JM, Rodríguez-Palenzuéla P (1998) Plant defense peptides. Biopolymers 47:479–491. https://doi.org/10.1002/(sici)1097-0282(1998)47:6%3c479::aid-bip6%3e3.0.co;2-k
Benko-Iseppon AM, Galdino SL, Calsa T Jr, Kido EA, Tossi A, Belarmino LC, Crovella S (2010) Overview on plant antimicrobial peptides. Curr Protein Pept Sci 11:181–188. https://doi.org/10.2174/138920310791112075
Li J, Hu S, Jian W, Xie C, Yang X (2021) Plant antimicrobial peptides: Structures, functions, and applications. Bot Stud 62:5. https://doi.org/10.1186/s40529-021-00312-x
Carvalho AO, Gomes VM (2011) Plant defensins and defensin–like peptides – Biological activities and biotechnological applications. Curr Pharm Des 17:4270–4293. https://doi.org/10.2174/138161211798999447
Carvalho AO, Gomes VM (2007) Role of plant lipid transfer proteins in plant cell physiology - A concise review. Peptides 28:1144–1153. https://doi.org/10.1016/j.peptides.2007.03.004
Daly NL, Rosengren KJ, Craik DJ (2009) Discovery, structure and biological activities of cyclotides. Adv Drug Deliv Rev 61:918–930. https://doi.org/10.1016/j.addr.2009.05.003
Bohlmann H, Apel K (1991) Thionins. Annu Rev Plant Physiol Plant Mol Biol 42:227–240. https://doi.org/10.1146/annurev.pp.42.060191.001303
Slavokhotova AA, Shelenkov AA, Andreev YA, Odintsova TI (2017) Hevein–like antimicrobial peptides of plants. Biochemistry 82:1659–1674. https://doi.org/10.1134/s0006297917130065
Gracy J, Le-Nguyen D, Gelly J-C, Kaas Q, Chiche L (2007) KNOTTIN: the knottin or inhibitor cystine knot scaffold in 2007. Nucleic Acids Res 36:D314–D319. https://doi.org/10.1093/nar/gkm939
Oliveira-Lima M, Benko-Iseppon AM, Neto JRCF, Rodriguez-Decuadro S, Kido EA, Crovella S, Pandolfi V (2017) Snakin: Structure, roles and applications of a plant antimicrobial peptide. Curr Protein Pept Sci 18:368–374. https://doi.org/10.2174/1389203717666160619183140
Sels J, Mathys J, De Coninck BMA, Cammue BPA, De Bolle MFC (2008) Plant pathogenesis–related (PR) proteins: A focus on PR peptides. Plant Physiol Biochem 46:941–950. https://doi.org/10.1016/j.plaphy.2008.06.011
Soares JR, Carvalho AO, Santos IS, Machado OLT, Nascimetno VV, Vasconcelos IM, Ferreria ATS, Perales JEA, Gomes VM (2012) Antimicrobial peptides from Adenanthera pavonina L. seeds: Characterization and antifungal activity. Protein Pept Lett 19:520–529. https://doi.org/10.2174/092986612800191062
Soares JR, Tenório J, de Melo E, da Cunha M, Fernandes KVS, Taveira GB, Pereira LS, Pimenta S, Trindade FG, Regente M, Pinedo M, de la Canal L, Gomes VM, Carvalho AO (2017) Interaction between the plant ApDef1 defensin and Saccharomyces cerevisiae results in yeast death through a cell cycle- and caspase-dependent process occurring via uncontrolled oxidative stress. Biochim Biophys Acta Gen Subj 1861:3429–3443. https://doi.org/10.1016/j.bbagen.2016.09.005
Maurycyo SG, Roberta CM, Hugo LMB, Aquino SR, Oliveira FCE, Islam MT, Pessoa CA, Rizzo MS, Costa MP (2020) Advances in the research of Adenanthera pavonina: From traditional use to intellectual property. J Med Plant Res 14:24–53. https://doi.org/10.5897/jmpr2019.6872
de Godoi AM, Faccin-Galhardi LC, Lopes N, Rechenchoski DZ, Almeida RR, Ricardo NMPS, Nozawa C, Linhares REC (2014) Antiviral activity of sulfated polysaccharide of Adenanthera pavonina against Poliovirus in HEp-2 cells. Evid Based Complement Alternat Med 2014:1–6. https://doi.org/10.1155/2014/712634
Benjamin S, Pinto Vieira I, Mendes FP, Silva SC, PAim RTT, Silva BB, Benjamin SR, Florean ET, Guedes MF (2018) Antidiabetic effects of galactomannans from Adenanthera pavonina L. in streptozotocin-induced diabetic mice. Asian Pac J Trop Med 11:116. https://doi.org/10.4103/1995-7645.225018
Hussain A, Rizvi A, Wahab S, Ansari S (2011) Antibacterial screening of the bark of Adenanthera pavonina (L.). Int J Biomed Res 2:110–122. https://doi.org/10.7439/ijbr.v2i2.85
Meriño-Cabrera Y, Oliveira Mendes TA, Castro JGS, Barbosa SL, Macedo MLR, Oliveira MGA (2020) Noncompetitive tight-binding inhibition of Anticarsia gemmatalis trypsins by Adenanthera pavonina protease inhibitor affects larvae survival. Arch Insect Biochem Physiol 104:e21687. https://doi.org/10.1002/arch.21687
Olukayode OJ, Emmanuel AO, Olajide AO, Makinde MJ (2009) Anticonvulsant and depressant activities of the seed extracts of Adenanthera pavonina. J Nat Prod 2:74–80
Ara A, Arifuzzaman M, Ghosh CK, Hashem MA, Ahmad MU, Bachar SC, Nahar L, Sarkerv SD (2010) Anti-inflammatory activity of Adenanthera pavonina L., Fabaceae, in experimental animals. Rev Bras Farmacogn 20:929–932. https://doi.org/10.1590/s0102-695x2010005000039
Afolabi IS, Nwachukwu IC, Ezeoke CS, Woke RC, Adegbite OA, Olawole TD (2018) Production of a new plant-based milk from Adenanthera pavonina seed and evaluation of its nutritional and health benefits. Front Nutr 5:9. https://doi.org/10.3389/fnut.2018.00009
Soysa P, Silva I (2011) Evaluation of phytochemical composition and antioxidant capacity of a decoction containing Adenanthera pavonina L. and Thespesia populnea L. Pharmacogn Mag 7:193. https://doi.org/10.4103/0973-1296.84229
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379. https://doi.org/10.1016/0003-2697(87)90587-2
Broekaert W (1990) An automated quantitative assay for fungal growth inhibition. FEMS Microbiol Lett 69:55–59. https://doi.org/10.1016/0378-1097(90)90412-j
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Moodley S, Koorbanally NA, Moodley T, Ramjugernath D, Pillay M (2014) The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay is a rapid, cheap, screening test for the in vitro anti-tuberculous activity of chalcones. J Microbiol Methods 104:72–78. https://doi.org/10.1016/j.mimet.2014.06.014
Macedo MLR, Garcia VA, Freire M, das GM, Richardson M, (2007) Characterization of a Kunitz trypsin inhibitor with a single disulfide bridge from seeds of Inga laurina (SW.) Willd. Phytochemistry 68:1104–1111. https://doi.org/10.1016/j.phytochem.2007.01.024
Kakade ML, Rackis JJ, McGhee JE, Puski G (1973) Determination of trypsin inhibitor activity of soy products: A collaborative analysis of an improved procedure. Am Assoc Cereal Chem 51:376–382
Liu D, Zeng X-A, Sun D-W, Han Z (2013) Disruption and protein release by ultrasonication of yeast cells. Innov Food Sci Emerg Technol 18:132–137. https://doi.org/10.1016/j.ifset.2013.02.006
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Silva FCV, Nascimento VV, Fernandes KV, Machado OLT, Pereira LS, Gomes VM, Carvalho AO (2018) Recombinant production and α-amylase inhibitory activity of the lipid transfer protein from Vigna unguiculata (L. Walp.) seeds. Process Biochem 65:205–212. https://doi.org/10.1016/j.procbio.2017.10.018
Burgess RR (2009) Protein precipitation techniques. Methods Enzymol 463:331–342. https://doi.org/10.1016/S0076-6879(09)63020-2
Duong-Ly KC, Gabelli SB (2014) Salting out of proteins using ammonium sulfate precipitation. Methods Enzymol 541:85–94. https://doi.org/10.1016/B978-0-12-420119-4.00007-0
Wingfield PT (2016) Protein precipitation using ammonium sulfate. Curr Protoc Protein Sci 84. https://doi.org/10.1002/0471140864.psa03fs84
Harcum S (2008) Purification of protein solutions. In: Biologically Inspired Textiles. Elsevier, p 26–43
Grant GA (2016) Isolation/purification of proteins. In: Encyclopedia of Cell Biology. Elsevier, p 66–74
Srivastava S, Dashora K, Ameta KL, Singh NP, El-Enshasy HA, Pagano MC, Hesham AE-L, Sharma GD, Sharma M, Bhargava A (2021) Cysteine-rich antimicrobial peptides from plants: The future of antimicrobial therapy. Phytother Res 35:256–277. https://doi.org/10.1002/ptr.6823
Lewis JS II, Graybill JR (2008) Fungicidal versus fungistatic: What’s in a word? Expert Opin Pharmacother 9:927–935. https://doi.org/10.1517/14656566.9.6.927
Walls D, Loughran ST (2017) Protein chromatography: Methods and protocols. Springer, New York, New York, NY
Boysen RI, Hearn MTW (2001) HPLC of peptides and proteins. Curr Protoc Protein Sci 8:8.7.1–8.7.40. https://doi.org/10.1002/0471140864.ps0807s23
Malik E, Dennison S, Harris F, Phoenix D (2016) PH dependent antimicrobial peptides and proteins, their mechanisms of action and potential as therapeutic agents. Pharmaceuticals (Basel) 9:67. https://doi.org/10.3390/ph9040067
Pabst TM, Carta G, Ramasubramanyan N, Hunter AK, Mensah P, Gustafson ME (2008) Separation of protein charge variants with induced pH gradients using anion exchange chromatographic columns. Biotechnol Prog 24:1096–1106. https://doi.org/10.1002/btpr.53
Games PD, Santos IS, Mello ÉO, Diz MSS, Carvalho AO, de Souza-Filho GA, Da Cunha M, Vasconcelos IM, Ferreira BS, Gomes VM (2008) Isolation, characterization and cloning of a cDNA encoding a new antifungal defensin from Phaseolus vulgaris L. seeds. Peptides 29:2090–2100. https://doi.org/10.1016/j.peptides.2008.08.008
Tang SS, Prodhan ZH, Biswas SK, Le CF, Sekaran SD (2018) Antimicrobial peptides from different plant sources: Isolation, characterization, and purification. Phytochemistry 154:94–105. https://doi.org/10.1016/j.phytochem.2018.07.002
Gebara RS, Taveira GB, Santos LA, Calixto SD, Simão TLBV, Lassounskaia E, Muzitano MF, Teixeira-Ferreira A, Perales J, Rodrigues R, Carvalho AO, Gomes VM (2020) Identification and characterization of two defensins from Capsicum annuum fruits that exhibit antimicrobial activity. Probiotics Antimicrob Proteins 12:1253–1265. https://doi.org/10.1007/s12602-020-09647-6
Arranz-Trullén J, Lu L, Pulido D, Bhakta S, Boix E (2017) Host antimicrobial peptides: The promise of new treatment strategies against tuberculosis. Front Immunol 8:1–17. https://doi.org/10.3389/fimmu.2017.01499
Zhang Q-Y, Yan Z-B, Meng YM, Hong X-Y, Shao G, Ma J-J, Cheng X-R, Liu J, Kang J, Fu CY (2021) Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil Med Res 8:1–25. https://doi.org/10.1186/s40779-021-00343-2
Singh T, Choudhary P, Singh S (2022) Antimicrobial peptides: Mechanism of action, in: Insights Antimicrob Pept, IntechOpen, 2022. https://doi.org/10.5772/intechopen.99190
Mwangi J, Hao X, Lai R, Zhang Z-Y (2019) Antimicrobial peptides: New hope in the war against multidrug resistance. Zool Res 40:488–505. https://doi.org/10.24272/j.issn.2095-8137.2019.062
Terras FRG, Schoofs ME, De Bolle MFC, Van Leuven F, Rees SB, Vanderleyden J, Cammue BP, Broekaert WF (1992) Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J Biol Chem 267:15301–15309
van der Weerden NL, Bleackley MR, Anderson MA (2013) Properties and mechanisms of action of naturally occurring antifungal peptides. Cell Mol Life Sci 70:3545–3570. https://doi.org/10.1007/s00018-013-1260-1
McGregor D (2008) Discovering and improving novel peptide therapeutics. Curr Opin Pharmacol 8:616–619. https://doi.org/10.1016/j.coph.2008.06.002
Abedinzadeh M, Gaeini M, Sardari S (2015) Natural antimicrobial peptides against Mycobacterium tuberculosis. J Antimicrob Chemother 70:1285–1289. https://doi.org/10.1093/jac/dku570
Prabhu KS, Pattabiraman TN (1980) Natural plant enzyme inhibitors. Isolation and characterization of a trypsin/chymotrypsin inhibitor from Ideina red wood (Adenanthera pavonina) seeds. J Sci Food Agric 31:967–980
Richardson M, Campos FAP, Xavier-Filho J, Macedo MLR, Maia GMC, Yarwood A (1996) The amino acid sequence and reactive (inhibitory) site of the major trypsin isoinhibitor (DE5) isolated from seeds of the Brazilian Carolina tree (Adenanthera pavonina L.). Biochim Biophys Acta 872:134–140. https://doi.org/10.1016/0167-4838(86)90156-1
Macedo MLR, Durigan RA, Silva DS, Marangoni S, Freire MGF, Parra JRP (2010) Adenanthera pavonina trypsin inhibitor retard growth of Anagasta kuehniella (Lepidoptera: Pyralidae). Arch Insect Biochem Physiol 73:213–231. https://doi.org/10.1002/arch.20352
Macedo MLR, Sá CM, Freire MGM, Parra JRP (2004) A Kunitz-type inhibitor of coleopteran proteases, isolated from Adenanthera pavonina L. seeds and its effect on Callosobruchus maculates. J Agric Food Chem 52:2533–2540. https://doi.org/10.1021/jf035389z
Migliolo L, Oliveira AS, Santos EA, Franco OL, Sales MP (2010) Structural and mechanistic insights into a novel noncompetitive Kunitz trypsin inhibitor from Adenanthera pavonina L. seeds with double activity toward serine– and cysteine–proteinases. J Mol Graph Model 29:148–156. https://doi.org/10.1016/j.jmgm.2010.05.006
Souza DD, Brandão-Costa RMP, Albuquerque WWC, Porto ALF (2016) Partial purification and characterization of a trypsin inhibitor isolated from Adenanthera pavonina L. seeds. S Afr J Bot 104:30–34. https://doi.org/10.1016/j.sajb.2015.11.008
Chandrashekharaiah KS, Shashank A, Bharadwaj R, Raju NG, Swamy NR (2017) Characterization of protease inhibitors from the seeds of Adenanthera pavonina. Int J Appl Biotechnol Biochem 13:361–370
Krishnan HB, Kim S, Pereira AE, Jurkevich A, Hibbard BE (2022) Adenanthera pavonina, a potential plant-based protein resource: Seed protein composition and immunohistochemical localization of trypsin inhibitors. Food Chem X 13:100253. https://doi.org/10.1016/j.fochx.2022.100253
Sruthi CK, Prakash M (2018) Amino acid impact factor. PLoS ONE 13:e0198645. https://doi.org/10.1371/journal.pone.0198645
Joshi RS, Mishra M, Suresh CG, Gupta VS, Giri AP (2013) Complementation of intramolecular interactions for structural–functional stability of plant serine proteinase inhibitors. Biochim Biophys Acta Gen Subj 1830:5087–5094. https://doi.org/10.1016/j.bbagen.2013.07.019
Silva MS, Santos LA, Taveira GB, Nagano CS, Chaves RP, Carvalho AO, Rodrigues R, Gomes VM (2023) Trypsin/α–amylase inhibitors from Capsicum chinense seeds: Characterization and antifungal activity against fungi of agronomic importance. Protein Pept Lett 30:260–274. https://doi.org/10.2174/0929866530666230221141804
Kim J-Y, Park S-C, Kim M-H, Lim H-T, Park Y, Hahm K-S (2005) Antimicrobial activity studies on a trypsin–chymotrypsin protease inhibitor obtained from potato. Biochem Biophys Res Commun 330:921–927. https://doi.org/10.1016/j.bbrc.2005.03.057
Menegatti E, Tedeschi G, Ronchi S, Bortolotti F, Ascenzi P, Thomas RM, Bolognesi M, Palmieri S (1992) Purification, inhibitory properties and amino acid sequence of a new serine proteinase inhibitor from white mustard (Sinapis alba L.) seed. FEBS Lett 301:10–14. https://doi.org/10.1016/0014-5793(92)80199-q
Ascenzi P, Ruoppolo M, Amoresano A, Pucci P, Consonni R, Zetta L, Pascarella S, Bortolotti F, Menegatti E (1999) Characterization of low–molecular–mass trypsin isoinhibitors from oil–rape (Brassica napus var. oleifera) seed. Eur J Biochem 261:275–284. https://doi.org/10.1046/j.1432-1327.1999.00275.x
Diz MS, Carvalho AO, Ribeiro SFF, Da Cunha M, Beltramini L, Rodrigues R, Nascimento VV, Machado OLT, Gomes VM (2011) Characterization, immunolocalization and antifungal activity of a lipid transfer protein from chili pepper (Capsicum annuum) seeds with novel α–amylase inhibitory properties. Physiol Plant 142:233–246. https://doi.org/10.1111/j.1399-3054.2011.01464.x
Santos LA, Taveira GB, Ribeiro SFF, Pereira LS, Carvalho AO, Rodrigues R, Oliveira AEA, Machado OLT, Araújo JS, Vasconcelos IM, Gomes VM (2017) Purification and characterization of peptides from Capsicum annuum fruits which are α-amylase inhibitors and exhibit high antimicrobial activity against fungi of agronomic importance. Protein Expr Purif 132:97–107. https://doi.org/10.1016/j.pep.2017.01.013
Agbowuro AA, Huston WM, Gamble AB, Tyndall JDA (2018) Proteases and protease inhibitors in infectious diseases. Med Res Rev 38:1295–1331. https://doi.org/10.1002/med.21475
Burchacka E, Pięta P, Łupicka-Słowik A (2022) Recent advances in fungal serine protease inhibitors. Biomed Pharmacother 146:112523. https://doi.org/10.1016/j.biopha.2021.112523
Pomarico L, Cerqueira DF, Soares RMA, Souza IPR, Castro GFBA, Socransky S, Haffajee A, Teles RP (2009) Associations among the use of highly active antiretroviral therapy, oral candidiasis, oral Candida species and salivary immunoglobulin A in HIV–infected children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:203–210. https://doi.org/10.1016/j.tripleo.2009.05.008
Berman J, Krysan DJ (2020) Drug resistance and tolerance in fungi. Nat Rev Microbiol 18:319–331. https://doi.org/10.1038/s41579-019-0322-2
Food and Agriculture Organization of the United Nations (2017) Strategic work of FAO for sustainable food and agriculture. 6488EN/1/01.17
Yactayo-Chang JP, Tang HV, Mendoza J, Christensen SA, Block AK (2020) Plant defense chemicals against insect pests. Agronomy 10:1156. https://doi.org/10.3390/agronomy10081156
Arya GC, Sarkar S, Manasherova E, Aharoni A, Cohen H (2021) The plant cuticle: An ancient guardian barrier set against long-standing rivals. Front Plant Sci 12:663165. https://doi.org/10.3389/fpls.2021.663165
Møller MS, Svensson B (2022) Structure, function and protein engineering of cereal-type inhibitors acting on amylolytic enzymes. Front Mol Biosci 9:868568. https://doi.org/10.3389/fmolb.2022.868568
Chrispeels MJ, Sá MFG, Higgins TJV (1998) Genetic engineering with α–amylase inhibitors makes seeds resistant to bruchids. Seed Sci Res 8:257–264. https://doi.org/10.1017/S0960258500004153
Luo M, Wang Z, Li H, Xia K-F, Cai Y, Xu Z-F (2009) Overexpression of a weed (Solanum americanum) proteinase inhibitor in transgenic tobacco results in increased glandular trichome density and enhanced resistance to Helicoverpa armigera and Spodoptera litura. Int J Mol Sci 10:1896–1910. https://doi.org/10.3390/ijms10041896
Wallace BA (2009) Protein characterization by synchrotron radiation circular dichroism spectroscopy. Q Rev Biophys 42(4):317–370. https://doi.org/10.1017/S003358351000003X
Cotabarren J, Lufrano D, Parisi MG, Obregón WD (2020) Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. Plant Sci 292:110398. https://doi.org/10.1016/j.plantsci.2019.110398
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brasil—(CAPES)—Finance Code 001. We acknowledge the financial support of the Brazilian agencies CNPq and FAPERJ (E-26/202.760/2018-Bolsa). We acknowledge Luiz Carlos Dutra de Souza and Valeria Miguelote Kokis for their technical support.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Finance Code 001, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, E-26/202.760/2018-Bolsa.
Author information
Authors and Affiliations
Contributions
Gebara RS conceived, performed and wrote the manuscript. Silva MS performed the enzyme inhibition assays. Calixto SD performed the cytotoxicity assay. Simão TLBV performed the antimycobacterial assay. Zeraik AE performed the circular dichroism assay, Elena Lassounskaia conceived the antimycobacterial assay. Muzitano MF conceived the cytotoxicity assay. Petretski JH conceived the zymography analysis. Gomes VM revised the manuscript. Carvalho AO conceived and designed the experiments, acquired the fundings, and wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• ApPI, a new thermally stable serine protease/α‒amylase inhibitor from A. pavonina.
• ApPI inhibits Candida albicans and Mycobacterium tuberculosis.
• ApPI inhibits serine proteases extracted from Saccharomyces cerevisiae and C. albicans.
• ApPI retains antimicrobial activity after heating at 130 °C for 30 min.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva Gebara, R., da Silva, M.S., Calixto, S.D. et al. Antifungal, Antimycobacterial, Protease and α‒Amylase Inhibitory Activities of a Novel Serine Bifunctional Protease Inhibitor from Adenanthera pavonina L. Seeds. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10194-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10194-z