Abstract
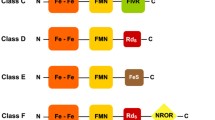
Escherichia coli RclA and Staphylococcus aureus MerA are part of the Group I flavoprotein disulfide reductase (FDR) family and have been implicated in the contribution to bacterial pathogenesis by defending against the host immune response. Fusobacterium nucleatum is a pathogenic, anaerobic Gram-negative bacterial species commonly found in the human oral cavity and gastrointestinal tract. In this study, we discovered that the F. nucleatum protein FN0820, belonging to the Group I FDR family, exhibited a higher activity of a Cu2+-dependent NADH oxidase than E. coli RclA. Moreover, FN0820 decreased the dissolved oxygen level in the solution with higher NADH oxidase activity. We found that L-tryptophan and its analog 5-hydroxytryptophan inhibit the FN0820 activities of NADH oxidase and the concomitant reduction of oxygen. Our results have implications for developing new treatment strategies against pathogens that defend the host immune response with Group I FDRs.







Similar content being viewed by others
Data availability
The data underlying the results are available as part of the article and further data are available from the corresponding author on reasonable request.
References
Baek, Y., Kim, J., Ahn, J., Jo, I., Hong, S., Ryu, S., & Ha, N. C. (2020). Structure and function of the hypochlorous acid–induced flavoprotein RclA from Escherichia coli. Journal of Biological Chemistry, 295, 3202–3212.
Basic, A., Blomqvist, M., Dahlén, G., & Svensäter, G. (2017). The proteins of Fusobacterium spp. involved in hydrogen sulfide production from L-cysteine. BMC Microbiology, 17, 61.
Brennan, C. A., & Garrett, W. S. (2019). Fusobacterium nucleatum—symbiont, opportunist and oncobacterium. Nature Reviews Microbiology, 17, 156–166.
Derke, R. M., Barron, A. J., Billiot, C. E., Chaple, I. F., Lapi, S. E., Broderick, N. A., & Gray, M. J. (2020). The Cu (II) reductase RclA protects Escherichia coli against the combination of hypochlorous acid and intracellular copper. mBio, 11, e01905–20.
Diaz, P. I., Zilm, P. S., & Rogers, A. H. (2000). The response to oxidative stress of Fusobacterium nucleatum grown in continuous culture. FEMS Microbiology Letters, 187, 31–34.
Diaz, P. I., Zilm, P. S., & Rogers, A. H. (2002). Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology, 148, 467–472.
Guo, L., Shokeen, B., He, X., Shi, W., & Lux, R. (2017). Streptococcus mutans SpaP binds to RadD of Fusobacterium nucleatum ssp. polymorphum. Molecular Oral Microbiology, 32, 355–364.
Gursoy, U. K., Pöllänen, M., Könönen, E., & Uitto, V. J. (2010). Biofilm formation enhances the oxygen tolerance and invasiveness of Fusobacterium nucleatum in an oral mucosa culture model. Journal of Periodontology, 81, 1084–1091.
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., Tunyasuvunakool, K., Bates, R., Žídek, A., Potapenko, A., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature, 596, 583–589.
Kleiger, G., & Eisenberg, D. (2002). GXXXG and GXXXA motifs stabilize FAD and NAD(P)-binding Rossmann folds through Cα–H···O hydrogen bonds and van der Waals interactions. Journal of Molecular Biology, 323, 69–76.
Ledwidge, R., Patel, B., Dong, A., Fiedler, D., Falkowski, M., Zelikova, J., Summers, A. O., Pai, E. F., & Miller, S. M. (2005). NmerA, the metal binding domain of mercuric ion reductase, removes Hg2+ from proteins, delivers it to the catalytic core, and protects cells under glutathione-depleted conditions. Biochemistry, 44, 11402–11416.
Meredith, J. D., Chapman, I., Ulrich, K., Sebastian, C., Stull, F., & Gray, M. J. (2022). Escherichia coli RclA is a highly active hypothiocyanite reductase. Proceedings of the National Academy of Sciences, 119, e2119368119.
Parker, B. W., Schwessinger, E. A., Jakob, U., & Gray, M. J. (2013). The RclR protein is a reactive chlorine-specific transcription factor in Escherichia coli. Journal of Biological Chemistry, 288, 32574–32584.
Schrödinger, L. (2010). The PyMOL molecular graphics system. Version 1.5.
Shearer, H. L., Loi, V. V., Weiland, P., Bange, G., Altegoer, F., Hampton, M. B., Antelmann, H., & Dickerhof, N. (2023). MerA functions as a hypothiocyanous acid reductase and defense mechanism in Staphylococcus aureus. Molecular Microbiology, 119, 456–470.
Shen, Y., & Buick, R. (2004). The antiquity of microbial sulfate reduction. Earth-Science Reviews, 64, 243–272.
Signat, B., Roques, C., Poulet, P., & Duffaut, D. (2011). Role of Fusobacterium nucleatum in periodontal health and disease. Current Issues in Molecular Biology, 13, 25–36.
Yoshida, Y., Ito, S., Kamo, M., Kezuka, Y., Tamura, H., Kunimatsu, K., & Kato, H. (2010). Production of hydrogen sulfide by two enzymes associated with biosynthesis of homocysteine and lanthionine in Fusobacterium nucleatum subsp. nucleatum ATCC 25586. Microbiology, 156, 2260–2269.
Yoshihara, T., Kioi, M., Baba, J., Usuda, H., Kessoku, T., Iwaki, M., Takatsu, T., Misawa, N., Ashikari, K., Matsuura, T., et al. (2021). A prospective interventional trial on the effect of periodontal treatment on Fusobacterium nucleatum abundance in patients with colorectal tumours. Scientific Reports, 11, 23719.
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (MSIT) (grant NRF-2019M3E5D6063871). This research was also supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET), funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (321036053HD020).
Author information
Authors and Affiliations
Contributions
YB and NCH designed the research. YB, JR, and JHP conceived and performed the experiments. SJ, IJ and NCH provided expertise and feedback. YB, JR, and NCH wrote the manuscript, and NCH secured funding.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no conflict of interest to report.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, H., Baek, Y., Lee, D. et al. Structural and Functional Analyses of the Flavoprotein Disulfide Reductase FN0820 of Fusobacterium nucleatum. J Microbiol. 61, 1033–1041 (2023). https://doi.org/10.1007/s12275-023-00095-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-023-00095-9