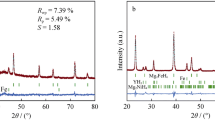
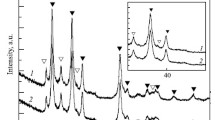
The method of reactive ball milling was used to synthesize MgH2-based composites adding nanoparticles of complex oxides RTO3 (R-rare earth and T-transition metals) as catalysts and graphite. All composites contain 5 wt.% of complex oxides Dy0.5Nd0.5FeO3 and TbFe0.5Cr0.5O3 synthesized by the sol-gel method, and some of them additionally contain 3 wt.% of graphite. The oxides have an orthorhombic perovskite structure (GdFeO3 type) and are characterized by an average particle size of 80–300 nm. The effect of perovskites on the hydrogenation of magnesium during the milling process and the improvement of hydrogen sorption-desorption kinetics is demonstrated. The Mg–Dy0.5Nd0.5FeO3 and Mg–TbFe0.5Cr0.5O3 composites absorbed 6.7 and 6.2 wt.% of hydrogen, respectively. X-ray powder diffraction after ball milling did not reveal any new compounds, except magnesium hydride. Thermal desorption from these composites occurs in two stages at temperatures above 300°C. The activation energy (Ea) of hydrogen desorption was determined by the Kissinger method. For the composite with TbFe0.5Cr0.5O3, Ea is 123 kJ/mol, and for the composite with Dy0.5Nd0.5FeO3 Ea = 147 kJ/mol. These composites were also tested as materials for hydrogen generation by hydrolysis in pure water and MgCl2 water solutions. In pure water, the hydrogen yield during hydrolysis ranged from 320 to 350 ml per gram. The conversion degree was significantly improved by the addition of MgCl2. It reached 90% (~1400 ml/g) after 30 min of hydrolysis for the MgH2–nano-TbFe0.5Cr0.5O3. These characteristics show that the synthesized MgH2–nano-RTO3 composites can be used in hydrogen generation systems.







Similar content being viewed by others
Change history
21 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11106-024-00411-x
References
V.A. Yartys, M.V. Lototskyy, E. Akiba, R. Albert, V.E. Antonov, J.R. Ares, M. Baricco, N. Bourgeois, C.E. Buckley, J.M. Bellosta von Colbe, J.-C. Crivello, F. Cuevas, R.V. Denys, M. Dornheim, M. Felderhoff, D.M. Grant, B.C. Hauback, T.D. Humphries, I. Jacob, T.R. Jensen, P.E. de Jongh, J.-M. Joubert, M.A. Kuzovnikov, M. Latroche, M. Paskevicius, L. Pasquini, L. Popilevsky, V.M. Skripnyuk, E. Rabkin, M.V. Sofianos, A. Stuart, G. Walker, H. Wang, C.J. Webb, and M. Zhu, “Magnesium based materials for hydrogen-based energy storage: Past, present and future,” Int. J. Hydrogen Energy, 44, 7809–7859 (2019).
A. Baran and M. Polanski, “Magnesium-based materials for hydrogen storage – A scope review,” Materials, 13, 3993 (2020).
I.Y. Zavalii, V.V. Berezovets, and R.V. Denys, “Nanocomposites based on magnesium for hydrogen storage: achievements and prospects (a survey),” Mater. Sci., 54, 611–626 (2019).
B. Sakintuna, F. Lamari-Darkrim, and M. Hirscher, “Metal hydride materials for solid hydrogen storage: A review,” Int. J. Hydrogen Energy, 32, 1121–1140 (2007).
M.V. Lototsky, R.V. Denys, and V.A. Yartys, “Combustion-type hydrogenation of nanostructured Mgbased composites for hydrogen storage,” Int. J. Energy Research., 33, 1114–1125 (2009).
G. Liang, J. Huot, S. Boily, A. Van Neste, and R. Schulz, “Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm = Ti, V, Mn, Fe, and Ni) systems,” J. Alloys Compd., 292, 247–252 (1999).
N. Hanada, T. Ichikawa, and H. Fujii, “Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling,” J. Phys. Chem. B, 109, 7188–7194 (2005).
T. Sadhasivam, H.T. Kim, S. Jung, S.H. Roh, J.H. Park, and H.Y. Jung, “Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review,” Ren. Sust. Energy Rev., 72, 523–534 (2017).
H. Gasan, O.N. Celik, N. Aydinbeyli, and Y.M. Yaman, “Effect of V, Nb, Ti and graphite additions on the hydrogen desorption temperature of magnesium hydride,” Int. J. Hydrogen Energy, 37, 1912–1918 (2012).
W. Oelerich, T. Klassen, and R. Bormann, “Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials,” J. Alloys Compd., 315, 237–242 (2001).
M.P. Pitt, M. Paskevicius, C.J. Webb, D.A. Sheppard, C.E. Buckley, A.E. MacGray, “The synthesis of nanoscopic Ti based alloys and their effects on the MgH2 system compared with the MgH2 + 0.01Nb2O5 benchmark,” Int. J. Hydrogen Energy, 37, 4227–4237 (2012).
G. Barkhordarian, T. Klassen, and R. Bormann, “Effect of Nb2O5 content on hydrogen reaction kinetics of Mg,” J. Alloys Compd., 364, 242–246 (2004).
M. Lototskyy, M.W. Davids, J.M. Sibanyoni, J. Goh, and B.G. Pollet, “Magnesium-based hydrogen storage nanomaterials prepared by high energy reactive ball milling in hydrogen at the presence of mixed titanium–iron oxide,” J. Alloys Compd., 645, S454–S459 (2015).
N. Hanada, T. Ichikawa, S. Isobe, T. Nakagawa, K. Tokoyoda, T. Honma, H. Fujii, and Y. Kojima, “X-ray absorption spectroscopic study on valence state and local atomic structure of transition metal oxides doped in MgH2,” J. Phys. Chem. C, 113, 13450–13455 (2009).
N. Patelli, M. Calizzi, A. Migliori, V. Morandi, and L. Pasquini, “Hydrogen desorption below 150°C in MgH2–TiH2 composite nanoparticles: Equilibrium and kinetic properties,” J. Phys. Chem. C, 121, 11166–11177 (2017).
L. Zhang, X. Lu, L. Ji, N. Yan, Z. Sun, and X. Zhu, “Catalytic effect of facile synthesized TiH1.971 nanoparticles on the hydrogen storage properties of MgH2,” Nanomaterials, 9, 1370 (2019).
V.V. Berezovets, R.V. Denys, I.Yu. Zavaliy, and Yu.V. Kosarchyn, “Effect of Ti-based nanosized additives on the hydrogen storage properties of MgH2, Int. J. Hydrogen Energy, 47, 7289–7298 (2022).
J. Lu, Y.J. Choi, Z.Z. Fang, H.Y. Sohn, and E. Ronnebro, “Hydrogen storage properties of nanosized MgH2–0.1TiH2 prepared by ultrahigh-energy-high pressure milling,” J. Am. Chem. Soc., 131, 15843–15852 (2009).
A. Patah, A. Takasaki, and J.S. Szmyd, “Influence of multiple oxides (Cr2O3/Nb2O5) addition on the sorption kinetics of MgH2,” Int. J. Hydrogen Energy, 4, 3032–3037 (2009).
M. Polanski and J. Bystrzycki, “Comparative studies of the influence of different nano-sized metal oxides on the hydrogen sorption properties of magnesium hydride,” J. Alloys Compd., 486, 697–701 (2009).
I.Yu. Zavaliy, V.V. Berezovets, I.V. Oshchapovsky, and T.M. Zasadnyy, M”g–TiN and Mg–ZrN nanocomposites as efficient materials for the accumulation and generation of hydrogen,” Mater. Sci., 57, 53–60 (2021).
I.Yu. Zavaliy, V.V. Berezovets, R.V. Denys, O.P. Kononiuk, and V.A. Yartys, “Hydrogen absorptiondesorption properties and hydrolysis performance of MgH2-Zr3V3O0.6Hx and MgH2–Zr3V3O0.6Hx–C composites,” J. Energy Storage, 65, 1072445 (2023).
V.V. Berezovets, O.P. Kononiuk, R.V. Denys, I.Yu. Zavaliy, “Synthesis and hydrogen-sorption properties of MgH2 composites with additions of TiFe intermetallics and its suboxide,” Physical-Chemical Mechanics of Materials, No. 2, 73–79 (2023).
M.S. Yahya and M. Ismail, “Catalytic effect of SrTiO3 on the hydrogen storage behavior of MgH2,” J. Energy Chemistry, 28, 46–53 (2019).
W. Zhang, Y. Cheng, Y. Li, Z. Duan, and J. Liu, “Effect of LaFeO3 on hydrogenation/dehydrogenation properties of MgH2,” J. Rare Earths, 33, 334–338 (2015).
N.A. Sazelee, N.H. Idris, M.F. Md Din, M.S. Yahya, N.A. Ali, and M. Ismail, “LaFeO3 synthesized by solid-state method for enhanced sorption properties of MgH2,” Results in Physics, 16, 102844 (2020).
Ch. Wu, Y. Wang, Y. Liu, W. Ding, and Ch. Sun, “Enhancement of hydrogen storage properties by in situ formed LaH3 and Mg2NiH4 during milling MgH2 with porous LaNiO3,” Catalysis Today, 318, 113–118 (2018).
D. Pukazhselvan, N. Nasani, T. Yang, D. Ramasamy, A. Shaula, and D.P. Fagg, “Chemically transformed additive phases in Mg2TiO4 and MgTiO3 loaded hydrogen storage system MgH2,” App. Surf. Sci., 472, 99–104 (2019).
M. Hilman, A. Rahman, M.A. Shamsudin, A. Klimkowicz, S. Uematsu, and A. Takasaki, “Effects of KNbO3 catalyst on hydrogen sorption kinetics of MgH2,” Int. J. Hydrogen Energy, 44, 29196–29202 (2019).
D. Pukazhselvan, N. Nasani, P. Correia, E. Carbo-Argibay, G. Otero-Irurueta, D.G. Stroppa, D.P. Fagg, “Evolution of reduced Ti containing phase(s) in MgH2/TiO2 system and its effect on the hydrogen storage behavior of MgH2,” J. Power Sources, 362, 174–183 (2017).
N.N. Sulaiman, N. Juahir, N.S. Mustafa, F.A. Halim Yap, and M. Ismail, “Improved hydrogen storage properties of MgH2 catalyzed with K2NiF6,” J. Energy Chemistry, 25, 832–839 (2016).
O. Pavlovska, I. Lutsyuk, A. Kondyr, Ya. Zhydachevskyy, Ya. Vakhula, A. Pieniazek, L. Vasylechko, “Synthesis and structure characterisation of micro- and nanocrystalline powders of Dy1–xRxFeO3 (R = La, Pr, Nd, Sm, Gd),” Acta Physica Polonica A, 133, 802–805 (2018).
V.V. Berezovets, A.R. Kytsya, I.Yu. Zavaliy, and V.A. Yartys, “Kinetics and mechanism of hydrolysis of MgH2 in MgCl2 solutions,” Int. J. Hydrogen Energy, 46, Article 40278 (2021).
V.A. Yartys, Yu.M. Solonin, and I.Yu. Zavaliy, Hydrogen Based Energy Storage: Status and Recent Developments, Kyiv (2021), 268 p.
I.Y. Zavaliy, V.V. Berezovets, A.R. Kytsya, Yu.M. Solonin, and V.M. Kordan, “MgH2–ZrN composites for hydrogen generation by hydrolysis, Powder Metall. Met. Ceram., 60, 698–705 (2022).
Acknowledgments
The work was supported by the National Research Fund of Ukraine within the framework of grant No. 2020.02/0301 “Development of new functional materials for the needs of hydrogen energy.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Poroshkova Metallurgiya, Vol. 62, Nos. 5–6 (551), pp. 136–147, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kononiuk, O.P., Zavaliy, I.Y., Berezovets, V.V. et al. Catalytic Effect of RTO3 Perovskites on Hydrogen Storage and Hydrolysis Properties of Magnesium Hydride. Powder Metall Met Ceram 62, 372–381 (2023). https://doi.org/10.1007/s11106-023-00400-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-023-00400-6