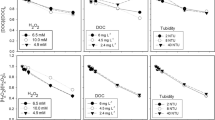
Abstract
The problem of water shortage is becoming increasingly serious. Looking for efficient and environmentally friendly water treatment technology has become the focus of today’s municipal engineering field. The ultraviolet-assisted electrocatalytic system is considered to be a new and efficient process. In this study, ultraviolet radiation was introduced into the self-made double-chamber dynamic diaphragm system electrolytic cell, and the synergistic effect of electrocatalytic reaction and ultraviolet radiation was used to achieve efficient degradation of rhodamine B. The effects of initial concentration, electrolytic voltage, and electrolyte concentration on the degradation efficiency were further studied. The optimal conditions were established by statistical methods such as the response surface method. After 60 min, the decolorization rate of rhodamine B in the positive and negative chambers of the electrolytic cell reached more than 98%. In addition, the intermediate products in the degradation process were analyzed by LC-MS to explore the degradation mechanism of rhodamine B. The experimental results show that the UV-assisted electrocatalytic membrane system process has potential in the field of water treatment and provides a new and efficient treatment scheme for solving the problem of water shortage.
Graphical Abstract














Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Q. Zeng, Y. Wang, F. Zan, S.K. Khanal, T. Hao, Biogenic sulfide for azo dye decolorization from textile dyeing wastewater. Chemosphere 283, 131158 (2021)
A.K.D. Alsukaibi, Various approaches for the detoxification of toxic dyes in wastewater. Processes 10(10), 1968 (2022)
H.M. Solayman, M.A. Hossen, A.A. Aziz, N.Y. Yahya, K.H. Leong, L.C. Sim, M.U. Monir, K.-D. Zoh, Journal of Environmental Chemical Engineering. J. Environ. Chem. Eng. 11(3), 109610 (2023)
T.H. Nguyen, T. Watari, M. Hatamoto, T. Setiadi, T. Yamaguchi, Enhanced decolorization of dyeing wastewater in a sponges-submerged anaerobic reactor. Chemosphere 279, 130475 (2021)
M.A.D.F. Alarcón, R.Y.A. Jarro, M.A. Ahmed, K.A.G. Bustos, D.A.P. Tanaka, R.T. Hilares, Intensification of Red-G dye degradation used in the dyeing of alpaca wool by advanced oxidation processes assisted by hydrodynamic cavitation. Ultrason. Sonochem. 89, 106144 (2022)
M. Guo, B. Yuan, Y. Sui, Y. Xiao, J. Dong, L. Yang, L. Bai, H. Yang, D. Wei, W. Wang, H. Chen, Rational design of molybdenum sulfide/tungsten oxide solar absorber with enhanced photocatalytic degradation toward dye wastewater purification. J. Colloid Interface Sci. 631, 33–43 (2023)
A.S. Adekunle, J.A.O. Oyekunle, L.M. Durosinmi, O. Saheed, T.A. Ajayeoba, O.F. Akinyele, S.E. Elugoke, O.S. Oluwafemi, Comparative photocatalytic degradation of dyes in wastewater using solar enhanced iron oxide (Fe2O3) nanocatalysts prepared by chemical and microwave methods. Nano-Struct. Nano-Objects 28(2021)
F.D. Castro, J.P. Bassin, M. Dezotti, Treatment of a simulated textile wastewater containing the reactive orange 16 azo dye by a combination of ozonation and moving-bed biofilm reactor: evaluating the performance, toxicity, and oxidation by-products. Environ. Sci. Pollut. Res. 24, 6307–6316 (2017)
A. Chaturvedi, R.P. Jaiswal, Optimization for minimizing the cost of ozonation of highly concentrated textile dyeing wastewater in a bubble column reactor. Environ. Sci. Pollut. Res. 29, 88018–88026 (2022)
S. Millan, L. Satish, S. Kesh, Y.S. Chaudhary, H. Sahoo, Interaction of lysozyme with rhodamine B: a combined analysis of spectroscopic & molecular docking, J. Photochem. Photobiol. B, Biol. 162, 248–257 (2016)
H. Rao, W. Qi, R. Su, Z. He, X. Peng, Mechanistic and conformational studies on the interaction of human serum albumin with rhodamine B by NMR, spectroscopic and molecular modeling methods. J. Mol. Liq. 316, 113889 (2020)
J. Shi, L. Gong, T. Zhang, S. Sun, Study of the seawater desalination performance by electrodialysis. Membranes 12(8), 767 (2022)
M.A. Alkhadra, T. Gao, K.M. Conforti, H. Tian, M.Z. Bazant, Small-scale desalination of seawater by shock electrodialysis. Desalination 476, 114219 (2020)
M. Nemati, S.M. Hosseini, M. Shabanian, Novel electrodialysis cation exchange membrane prepared by 2-acrylamido-2-methylpropane sulfonic acid; heavy metal ions removal. J. Hazard. Mater. 337, 90–104 (2017)
A. Zlotorowicz, R.V. Strand, O.S. Burheim, Ø. Wilhelmsen, S. Kjelstrup, The permselectivity and water transference number of ion exchange membranes in reverse electrodialysis. J. Membr. 523, 402–408 (2017)
Y. Hu, P.X. Mao, D.J. Cui, L. Xiong, P. Yin, S. Zhou, P.X. Wang, Electrochemical processes with a chambered dual-anode design for enhanced removal of dichlorophenol in aqueous solutions. ChemistrySelect 4(17), 5064–5072 (2019)
F. Ban, C. Ye, H. Nan, Optimization of dynamic diaphragm system by response surface methodology for synergistic electrochemical degradation of typical PPCPs wastewater. Pol. J. Environ. Stud. 32(3), 1–17 (2023)
S. Argote-Fuentes, R. Feria-Reyes, E. Ramos-Ramírez, N. Gutiérrez-Ortega, G. Cruz-Jiménez, Photoelectrocatalytic degradation of congo red dye with activated hydrotalcites and copper anode. Catalysts 11(2), 211 (2021)
H. Yin, Q. Zhang, J. Jing, X. Wang, X. Yin, M. Zhou, An efficient Fe2+ assisted UV/electrogenerated-chlorine process for carbamazepine degradation: the role of Fe(IV). Chemosphere 307, 136168 (2022)
B. Vahid, A. Khataee, Photoassisted electrochemical recirculation system with boron-doped diamond anode and carbon nanotubes containing cathode for degradation of a model azo dye. Electrochim. Acta 88, 614–620 (2013)
P.Y. Chan, M.G. El-Din, J.R. Bolton, A solar-driven UV/Chlorine advanced oxidation process. Water Res. 46(17), 5672–5682 (2012)
D. Wu, F. Li, Q. Chen, M. Wu, W. Duan, Q. Zhao, B. Pan, B. Xing, Mediation of rhodamine B photodegradation by biochar. Chemosphere 256, 127082 (2020)
Y. Yao, Q. Chen, J. Zhou, Influence of typical electrolytes on electrooxidation of bio-refractory reactive dye. J. Environ. Sci. 19, 1799–1810 (2022)
F.C. Moreira, R.A.R. Boaventura, E. Brillas, V.J.P. Vilar, Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl. Catal. B 202, 217–261 (2017)
A.V. Moreno-Palacios, R.E. Palma-Goyes, J. Vazquez-Arenas, R.A. Torres-Palma, Bench-scale reactor for cefadroxil oxidation and elimination of its antibiotic activity using electro-generated active chlorine. J. Environ. Chem. Eng. 7(3), 103173 (2019)
J.D. García-Espinoza, I. Robles, A. Durán-Moreno, L.A. Godínez, Photo-assisted electrochemical advanced oxidation processes for the disinfection of aqueous solutions: a review. Chemosphere 274, 129957 (2021)
A.A. Prakash, K. Sathishkumar, M.S. AlSalhi, S. Devanesan, P. Mani, S. Kamala-Kannan, S. Vijayanand, A. Rajasekar, Integrated approach of photo-assisted electrochemical oxidation and sequential biodegradation of textile effluent. Environ. Pollut. 307, 119412 (2022)
S. Abilaji, K. Sathishkumar, J. Narenkumar, M.S. Alsalhi, S. Devanesan, P. Parthipan, B. Muthuraj, A. Rajasekar, Sequential photo electro oxidation and biodegradation of textile effluent: elucidation of degradation mechanism and bacterial diversity. Chemosphere 331, 138816 (2023)
E.V.d. Santos, C. Sáez, P. Cañizares, C.A. Martínez-Huitle, M.A. Rodrigo, UV assisted electrochemical technologies for the removal of oxyfluorfen from soil washing wastes. Chem. Eng. J. 318, 2–9 (2017)
H. Kusic, N. Koprivanac, L. Srsan, Azo dye degradation using Fenton type processes assisted by UV irradiation: a kinetic study. J. Photochem. Photobiol. A 181(2–3), 195–202 (2006)
J.D. García-Espinoza, M. Zolfaghari, P.M. Nacheva, Synergistic effect between ultraviolet irradiation and electrochemical oxidation for removal of humic acids and pharmaceuticals. Water Environ. Res. 34(2), 232–146 (2020)
C.A. Martínez-Huitle, M.A. Rodrigo, I. Sirés, O. Scialdone, Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem. Rev. 115(24), 13362–13407 (2015)
S. Hussain, J.R. Steter, S. Gul, A.J. Motheo, Photo-assisted electrochemical degradation of sulfamethoxazole using a Ti/Ru0.3Ti0.7O2 anode: mechanistic and kinetic features of the process, J. Environ. Manage 201, 153–162 (2017)
C.K. Remucal, D. Manley, Emerging investigators series: the efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environ. Sci. Water Res. Technol 2, 565–579 (2016)
F. Ban, H. Nan, Q. Jin, M. Dai, Response surface methodology for optimizing the degradation of methyl orange in aqueous solution by a diaphragm system that utilizes a cathode and anode coaction electrochemical method. Pol. J. Environ. Stud. 31(1), 1–13 (2022)
G. Pan, X. Jing, X. Ding, Y. Shen, S. Xu, W. Miao, Synergistic effects of photocatalytic and electrocatalytic oxidation based on a three-dimensional electrode reactor toward degradation of dyes in wastewater. J. Alloys Compd. 809, 151749 (2019)
R. Antonelli, G.R.P. Malpass, M.G.C.d. Silva, M.G.A. Vieira, Photo-assisted electrochemical degradation of ciprofloxacin using DSA® anode with NaCl electrolyte and simultaneous chlorine photolysis, J. Water Process. Eng. 47, 102698 (2022)
L. Tian, M. Zhu, L.-S. Zhang, L.-J. Zhou, J.-P. Fan, D.-S. Wu, J.-P. Zou, New insights on the role of NaCl electrolyte for degradation of organic pollutants in the system of electrocatalysis coupled with advanced oxidation processes. J. Environ. Chem. Eng. 10(3), 107414 (2022)
J. Luo, H. Zhang, Z. Li, Highly efficient degradation of phenol from wastewater via an electro-catalytic oxidation approach with a CeO2–CuO cathode. RSC Adv. 8(27), 15167–15172 (2018)
R. Mei, Q. Wei, C. Zhu, W. Ye, B. Zhou, L. Ma, Z. Yu, K. Zhou, 3D macroporous boron-doped diamond electrode with interconnected liquid flow channels: a high-efficiency electrochemical degradation of RB-19 dye wastewater under low current. Appl. Catal. B 245(15), 420–427 (2019)
H. Wang, B. Wang, Y. Peng, J.C. Crittenden, H. Pana, L. Wang, Correction: improved VRC-3R− model for bulk water residual chlorine decay in the UV/Cl2 process for a water distribution network. Environ. Sci. Water Res. Technol. 9(2), 308–330 (2023)
T. Rasheed, M. Bilal, H.M.N. Iqbal, S.Z.H. Shah, H. Hu, X. Zhang, Y. Zhou, TiO2/UV-assisted rhodamine B degradation: putative pathway and identification of intermediates by UPLC/MS. Environ. Technol. 39(12), 1533–1543 (2018)
T. Hadibarata, R.A. Kristanti, Effect of environmental factors in the decolorization of remazol brilliant blue R by Polyporus sp. S133, J. Am. Chem. Soc. 57, 1095–1098 (2012)
Funding
This study was supported by the National Water Pollution and Control Major Project: Simulation and verification of key and integrated technologies for water pollution control in Liaohe River Basin (2018ZX07601001-3).
Author information
Authors and Affiliations
Contributions
In this research work, FB and CY participated in the design and operation of experiments and the preparation of papers, and HW was responsible for a proofreading and theoretical derivation; GL, TG, AX, and YW assisted and measured the experimental operation. All authors agree on the final version of the paper.
Corresponding author
Ethics declarations
Ethical Approval
This study is based on electrochemical experiments and does not involve human subject research or animal testing. The samples and data used in the experiment comply with ethical guidelines and policies. The process of obtaining and processing samples strictly complies with privacy protection and data confidentiality regulations. This study only used publicly available data and chemical reagents for experiments and did not involve any ethical issues.
Consent to Participate
After clearly understanding the research purpose, methods, and risks, participants agree to participate in this study.
Consent for Publication
The research participants have explicitly agreed that their data and results will be used for publishing papers.
Competing Interests
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ban, F., Ye, C., Wang, H. et al. Degradation of Rhodamine B by UV-Assisted Dynamic Diaphragm Electrocatalytic System: Efficiency Improvement and Mechanism Study. Electrocatalysis 15, 128–142 (2024). https://doi.org/10.1007/s12678-023-00862-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-023-00862-7