Abstract
Introduction
The NIH All of Us Research Program has enrolled over 544,000 participants across the US with unprecedented racial/ethnic diversity, offering opportunities to investigate myriad exposures and diseases. This paper aims to investigate the association between PM2.5 exposure and cancer risks.
Materials and methods
This work was performed on data from 409,876 All of Us Research Program participants using the All of Us Researcher Workbench. Cancer case ascertainment was performed using data from electronic health records and the self-reported Personal Medical History questionnaire. PM2.5 exposure was retrieved from NASA’s Earth Observing System Data and Information Center and assigned using participants’ 3-digit zip code prefixes. Multivariate logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (CI). Generalized additive models (GAMs) were used to investigate non-linear relationships.
Results
A total of 33,387 participants and 46,176 prevalent cancer cases were ascertained from participant EHR data, while 20,297 cases were ascertained from self-reported survey data from 18,133 participants; 9,502 cancer cases were captured in both the EHR and survey data. Average PM2.5 level from 2007 to 2016 was 8.90 μg/m3 (min 2.56, max 15.05). In analysis of cancer cases from EHR, an increased odds for breast cancer (OR 1.17, 95% CI 1.09–1.25), endometrial cancer (OR 1.33, 95% CI 1.09–1.62) and ovarian cancer (OR 1.20, 95% CI 1.01–1.42) in the 4th quartile of exposure compared to the 1st. In GAM, higher PM2.5 concentration was associated with increased odds for blood cancer, bone cancer, brain cancer, breast cancer, colon and rectum cancer, endocrine system cancer, lung cancer, pancreatic cancer, prostate cancer, and thyroid cancer.
Conclusions
We found evidence of an association of PM2.5 with breast, ovarian, and endometrial cancers. There is little to no prior evidence in the literature on the impact of PM2.5 on risk of these cancers, warranting further investigation.
Similar content being viewed by others
Introduction
Despite decades of improvements in ambient air quality in the United States [1], air pollution remains an environmental exposure of significant interest given disproportionate exposure [2,3,4] and the impact of even relatively low exposures on health [5] and health outcomes [6,7,8]. There is an ample evidence of its adverse impact on cardiovascular health [9, 10] and excess mortality [9, 11, 12]. The impact of poor air quality has also been extensively studied for lung cancer [9, 13,14,15,16,17], and associations with cancer have been observed at other organ sites; however, the epidemiological evidence is limited [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Outdoor air pollution and airborne particulate matter are classified as carcinogenic to humans for lung cancer [31], and evidence points to the need for further investigation of air quality’s impact on cancers including those of the bladder, breast, brain, liver, and kidney [34,35,36].
The All of Us Research Program is enrolling a cohort of over one million participants, offering researchers an unprecedented opportunity to investigate diseases including cancers [37, 38]. Notably, All of Us includes participants from racial and ethnic minority groups that have been underrepresented in previous cancer research cohorts [39]. All of Us may therefore confer sufficient statistical power to understand the burden of cancer in these populations and identify opportunities for intervention. In the era of precision prevention and precision medicine, investigating the role of the environment in cancer risk is critical [40,41,42]. Realizing the potential of precision health will call for holistic measures of individual risk that take the physical environment into account.
We recently conducted a preliminary investigation of cancer in the All of Us Research Program [43] as part of a demonstration project to show the quality, usefulness, validity, and diversity of the All of Us data [44]. We generated descriptive statistics for the most common cancers and considered differences in cancer case ascertainment compared to what would be expected in the broader US population by data source type (self-reported cancer in survey data and/or from the electronic health record). We found that over 13,000 cancer cases were self-reported in the study population of 315,000 people and nearly 24,000 cancer cases were detected in the electronic health records collected for All of Us research participants.
Researchers currently have access to data from 409,876 All of Us participants through the Researcher Workbench, including residential data for linkage to air pollution exposure. Although the program does not target enrollment by health status, the sample includes sufficient participants with a history of cancer, prevalent cancers, and incident cancers to enable initial investigation of the role of the environment on cancer in the All of Us Research Program. Here we investigate the association between ambient air pollution and any health outcome in All of Us for the first time, and we present preliminary findings on the association of air quality and cancer in this key precision medicine cohort. We focus on fine particulate matter (PM2.5), but our analysis suggests that this is only a first step toward understanding the full impact of diverse environmental factors on cancer and the extensive health outcomes collected by the All of Us Research Program.
Materials and methods
The All of Us Research Program
Data collected from 2017 to 2022 were accessed from the All of Us Research Program, a cohort of over 544,000 adults aged 18 and over living in the United States and its territories. The goals, recruitment methods and sites, and scientific rationale for All of Us have been described previously [37]. All of Us data include participants’ responses to a series of questionnaires, physical measurements collected by study staff at time of enrollment, and information from participants’ Electronic Health Records (EHR). These data are collected either at an All of Us affiliated health care provider organization (HPO) or through a “direct-volunteer” mechanism and are made available to researchers via the Researcher Workbench in registered, controlled, and restricted access tiers. Because zip code was required for this analysis, the data for this project were accessed at the controlled tier.
All of Us questionnaire data and physical measurements
Participant-provided information for our analysis including self-reported cancer diagnoses was derived from the Basics, Lifestyle, and Personal Medical History questionnaires. The full text of these questionnaires is available in the Survey Explorer found on the All of Us Research Hub, a publicly available website designed to support both researchers and the public [45]. The Basics questionnaire elicits demographic information including age, race/ethnicity, education, marital status, household income, and geography. The Lifestyle questionnaire collects data on the use of tobacco, alcohol, and other drugs. The Personal Medical History questionnaire collects self-reported cancer history. Age at cancer diagnosis in the survey is captured as child (0–11); adolescent (12–17); adult (18–64); older adult (65–74); and elderly (75+). The Basics and Lifestyle questionnaires are collected at baseline. Until recently, Personal Medical History was collected during retention efforts 3 months after enrollment; participants now have the option to complete this questionnaire at the time of enrollment. Body Mass Index (BMI) was calculated using participant height and weight collected by All of Us study staff at time of enrollment; height and weight data are housed in the Physical Measurements section of the Researcher Workbench.
EHR-derived cancer diagnoses
Cancer diagnosis data were also derived from participant electronic health records linked to their All of Us data. EHR-derived diagnoses were determined using Systematized Nomenclature of Medicine—Clinical Terms (SNOMED CT) codes and mapped to Observational Health and Medicines Outcomes Partnership (OMOP) concept ID by the All of Us Data and Research Center. EHR data include procedures, medications, laboratory tests, and health care provider visits. Our analysis used the following OMOP parent concept IDs for cancers/cancer sites: bladder: 93689003, 4095756, 4095755, 197508, 73712, 4312802; blood: 93143009, 109989006, 118601006; bone: 93725000, 78097; brain: 93727008, 4246451; breast: 372137005, 4157332, 4112853; cervix: 372024009, 198984; colon and rectum: 93761005, 36683531, 93984006, 435754, 4180790, 443382, 4180791, 4180792, 443390, 443381, 4181344, 443384; endocrine system: 4241776, 4156115, 371983001; endometrium: 4247238, 4095749; esophagus: 371984007, 4095316, 4094856, 4094854, 4181343, 4089656, 4092060, 4092059, 4094855; eye: 371986009; head, neck, and mouth: 372123001, 372001002, 4090224, 4177101, 4114222, 4089530, 25189, 4178964, 4181350, 4118989, 4090226; kidney: 93849006, 196653, 4091485; lung: 93880001, 443388, 4110587, 254591; ovary: 4116073, 4112864, 93934004, 4181351, 199752; pancreas 372003004, 4092072, 4112734, 4111024, 4178967, 4180793, 4095436; prostate: 93974005, 4163261; stomach: 372014001, 4095320, 4095319, 4149838, 4149837, 4092061, 4095317, 443387l; and thyroid: 94098005, 4178976, 36676291. Year of diagnosis was ascertained for cancer diagnosis, when available. Cases that first appear in the participant EHR after 2006 were included in analysis.
Air pollution exposure data
Daily PM2.5 concentrations were estimated at a resolution of 1 km × 1 km across the contiguous US using a well-validated ensemble-based prediction model that integrates random forest regression, gradient boosting machine, and artificial neural networking [46]. Over 100 variables were used for prediction in this approach including satellite data, land-use information, weather variables, and modeled chemical transport characteristics. We used a 10-year PM2.5 average from 2007 to 2016 for our exposure estimate. Output from this approach has been validated with daily PM2.5 concentrations measured at 2,156 US EPA monitoring sites. The validation results yielded an average cross-validated R-squared value of 0.86 for daily PM2.5 predictions, indicating outperformance compared to prior approaches [47, 48].
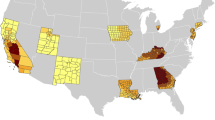
While residential addresses are not available in the All of Us Researcher Workbench, the dataset does contain 3-digit residential zip code prefix for each participant at enrollment. We therefore used zonal statistics to calculate the daily average PM2.5 concentration based on all 1 km × 1 km grids within the zip code. Specifically, we identified the 1 km × 1 km grids with centroid in one 3-digit zip code area and then averaged daily PM2.5 concentrations across all these grids. The average concentration was thus the PM2.5 exposure level for participants in that 3-digit zip code area. Figure 1 shows the distribution of All of Us participants represented in this analysis as well as the location of All of Us HPO sites.
Covariates
Following a review of known risk factors for cancer, we selected appropriate variables from the All of Us Researcher Workbench data for inclusion in all analyses. Baseline measurements of socioeconomic and demographic covariates including age (19–35, 36–50, 51–65, 65–89), sex at birth (female, male, other), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latino, Asian, other, multiracial, none of the above), current smoking status (yes, no), education (less than high school, high school graduate, some college, college graduate), and BMI (underweight, normal weight, overweight, obese) were included as covariates in the model.
Data analysis
Data were analyzed in the All of Us Researcher Workbench. The Researcher Workbench offers a secure environment and tools to enable users to select cohorts, create datasets for analysis, and conduct analysis using R and Python programming languages in a Jupyter Notebook. We generated descriptive statistics and prevalence for 19 cancers and conducted Chi-square tests to determine the difference in the categorical distribution of data source types (survey data, EHR, and both) across key categories. Descriptive analysis was undertaken on the prevalence of cancer as well as air pollution, and how these were distributed between the different groups of the covariates. To investigate the association between PM2.5 and cancer, univariable and multi-variable logistic regression were performed given the rare disease assumption and ability to approximate odds ratio from relative risk for interpretation convenience. Analyses were restricted to cases from the EHR to ensure that the diagnosis date did not occur prior to the exposure. We present the exposure distribution for cases obtained from different sources (EHR, survey, combined) but because date of diagnosis is not available in the survey, we did not include the survey data in logistic models. The first model introduced the unadjusted association between PM2.5 exposure and the outcome of interest (cancer overall and by type). The second model was adjusted for age, sex at birth, race/ethnicity, smoking status, education, and BMI. PM2.5 concentration was analyzed as a continuous variable as well as categorical variable (quartiles) in the regression models. To evaluate the non-linear relationship between PM2.5 exposure and cancer odds, we fitted a generalized additive model (GAM) including a spline term for the accessibility score with 3 degrees of freedom and visualized the exposure–outcome response with adjustment for other covariates. Participants with missing cancer data were excluded and missing values in covariates were treated as an independent category in the analysis. All analyses were conducted using the statistical software R version 4.2.1.
Results
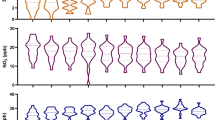
Table 1 shows the distribution of the mean annual PM2.5 exposure and the baseline characteristics of all participants (n = 409,876), among whom 42,462 participants had at least one self-reported or EHR-derived cancer diagnosis. Differences in age, sex at birth, race, smoking status, education, and BMI were observed between the participants overall, with older, female, Non-Hispanic White, non-smoking, more educated, and obese participants more likely to have data on cancer history. We also note differences in cancer outcomes by data source. 33,387 participants had at least one cancer in their EHR (337,292 participants had EHR data), and 18,133 participants reported at least one cancer in the Personal Medical History questionnaire (146,815 participants completed this questionnaire). 9,508 participants had at least one cancer in their EHR and in their Personal Medical History questionnaire responses. However, mean PM2.5 did not vary across these different populations. Figure 2 shows PM2.5 levels across the 862 3-digit zip code areas included in this analysis.
Table 2 shows that All of Us participants’ EHR data indicate a history of breast cancer most frequently (n = 8,433; 18.26% of cases) followed by blood cancers (n = 5,856; 12.68%), and prostate cancer (n = 5,322; 11.53%). More cancers were detected in the EHR passively as opposed to self-reported in the surveys, and the total case numbers are much lower (n = 9,502) for cancers cross-referenced in both the EHR and survey data. For the analysis of PM2.5 and cancer risk, the case population includes cases detected in the EHR (n = 46,176) with a diagnosis date after 2006. The number of cancer cases per participant is summarized in the supplemental table.
Table 3 presents cancer type distribution across the quartile distribution of PM2.5 exposure. More than 25% of blood, brain, breast, cervical, endometrial, and ovarian cancers are observed in the highest exposure quartile (10.67–15.05 µg/m3).
Table 4 reports the odds ratio (OR) and 95% confidence interval (CI) for air pollution with all cancers. The ORs are reported using the first quartile as the reference group. Comparing the highest quartile and lowest quartile of PM2.5, strong associations were observed for breast cancer (OR 1.17, 95% CI 1.09–1.25), endometrial cancer (OR 1.33, 95% CI 1.09–1.62), and ovarian cancer (OR 1.20, 95% CI 1.01–1.42). However, some inverse associations were also observed for bone cancer (4th vs.1st quartile: OR 0.78, 95% CI 0.69–0.88); colon and rectum cancer (4th vs. 1st quartile: OR 0.83, 95% CI 0.74–0.93); endocrine system cancer (4th vs. 1st quartile: OR 0.82, 95% CI 0.73–0.92); esophageal cancer (4th vs. 1st quartile: OR 0.55, 95% CI 0.40–0.76); eye cancer (4th vs. 1st quartile: OR 0.70, 95% CI 0.52–0.96), head and neck cancer (4th vs. 1st quartile: OR 0.89. 95% CI 0.82–0.98); lung cancer (4th vs. 1st quartile: OR 0.77, 95% CI 0.69–0.85); pancreatic cancer (4th vs. 1st quartile: OR 0.65, 95% CI 0.52–0.80); prostate cancer (4th vs. 1st quartile: OR 0.85, 95% CI 0.78–0.93); and stomach cancer (4th vs. 1st quartile: OR 0.69, 95% CI 0.53–0.91).
Sex and race stratified results are presented in Supplementary Tables 2 and 3. When stratified by sex, blood cancer is significant in males. The race/ethnicity stratified results show increases in blood cancer risk in NH Blacks and Asians as well as significant increases in bone, breast, and endometrial cancers among Hispanics. Asians demonstrated significantly increased risk in pancreatic cancer as well.
Figure 3 presents the non-linear relationship between PM2.5 and cancers with a p-value for spline less than 0.10. A non-linear relationship was observed for blood cancer, bone cancer, brain cancer, breast cancer, colon and rectum cancer, endocrine system cancer, lung cancer, pancreatic cancer, prostate cancer, and thyroid cancer. Notably, although we observed inverse associations for bone cancer, lung cancer, and pancreatic cancer in Table 4, results from GAM suggest that high PM2.5 concentrations increase the odds for these cancers.
Discussion
In this study, the mean PM2.5 concentration was 8.9 µg/m3, in line with the WHO health-based world air-quality guideline [49, 50]. The highest concentration of 15.1 µg/m3 was observed in California, while prior review reported an annual average PM2.5 concentration of 7.0 µg/m3 in the US [33]. The difference can be explained by the spatial distribution of our study population. At present, because urban residents have easier access to All of Us HPOs, most participants are concentrated in large cities such as New York City, Chicago, and Los Angeles where the level of air pollution is generally higher than rural areas. However, even the highest PM2.5 concentration in this study indicates a recent reduction in average PM2.5 exposure level across the US. For instance, a US-wide cohort study based on the American Cancer Society (ACS) Cancer Prevention Study II (CPS-II) reported a median PM2.5 concentration of 12.5 µg/m3 between 1999 and 2008, with the highest concentration of 28.0 µg/m3 [19].
Outdoor air pollution has been classified as Group 1 human carcinogens for lung cancer by the IARC since 2013 [31], a determination based largely on findings from outdoor air pollution exposure analysis in population cohort studies [14, 15]. Similarly, a recent meta-analysis reported a 9% increase in risk for lung cancer incidence or mortality per each 10 µg/m3 increase in PM2.5 concentration as well as an 8% (95% CI 0–17%) increase in risk per 10 µg/m3 for PM10 [16]. Our study observed an inverse association between PM2.5 and lung cancer. However, this inverse association was only manifest when the exposure level was low, which may reflect measurement error. In our analysis of the variables’ non-linear relationship, the odds for lung cancer increased when PM2.5 level exceeded a certain threshold. Therefore, our observation is still consistent with prior conclusions.
While the IARC has reported adverse associations between outdoor air pollution and bladder cancer [31, 51], this association was not observed in our study.
Systemic inflammation, oxidative stress, and epigenetic changes induced by PM exposure [52,53,54,55] are thought to play a role in the progression of breast tumors [56,57,58,59,60], and studies from a variety of settings demonstrate an association between PM2.5 levels and breast cancer mortality rates as well as all-cause mortality [56, 61]. A recent analysis of 47,433 women in the US Sister Study found adverse associations between PM2.5 (HR per 3.6 µg/m3, 1.05; 95% CI 0.99–1.11) and breast cancer incidence overall (n = 2,848) [22]. An analysis of 57,589 women in the Multiethnic Cohort observed adverse associations of NOx, NO2, PM2.5, and PM10 and breast cancer incidence among those living within 500 m of major roads [26]. The Canadian National Breast Screening Study (n = 89,247) found adverse associations of both PM2.5 (HR per 10 µg/m3, 1.26; 95% CI 0.99–1.61) and NO2 (HRs per 9.7 ppb, range 1.13–1.17) and the risk of incident premenopausal disease [62, 63]. However, no other recent studies have reported clear associations with incident breast cancer risk [23, 64, 65]. In our study, we did observe increased risk for breast cancer associated with PM2.5 exposure. This association was more evident when the PM2.5 level was high. The finding is generally consistent with previous studies that present suggestive associations for breast cancer. The larger number of breast cancer cases in this study yielded larger statistical power and may explain why we could observe associations in this study.
We also observed significant increased odds for endometrial and ovarian cancers. A recent study conducted in Beijing supports the gynecologic risks associated with air pollution [66]. However, in our study the mean PM2.5 concentration was lower than 10 µg/m3, a level in line with the World Health Organization (WHO) health-based world air-quality guideline [48, 49]. Our findings warrant further investigation of these cancers in air pollution studies.
A limitation of this study is that we only examined the association of PM2.5 with cancers while other pollutants such as SO2, NO2, NOx, and O3 were not included. PM2.5 is the most investigated pollutant and is often used as an indicator of overall air quality. However, the sole investigation of PM2.5 may lead to an underestimation of the association between air pollution and cancer risks. For instance, a recent review found that a higher risk of breast cancer was associated with NO2 and NOx, but not PM2.5 [60]. Another meta-analysis on leukemia concluded that higher exposure to NO2, but not PM2.5, was associated with higher leukemia risk. Additionally, this study only includes ambient PM2.5 exposure level and relies on historical data. A multi-level approach accounts for multiple pollutants and sources is warranted in future studies.
To preserve participant privacy, the All of Us Researcher Workbench only offers participant data at the 3-digit zip code prefix level, rather than at the full 5-digit level which would confer higher spatial resolution for exposure estimates. As the first three digits of a zip code designate a city or a larger rural area, exposure assessment in this study may underestimate geospatial variations in air pollution. Recent epidemiological research has demonstrated the importance of within-city variability in air pollution concentration [67, 68]. However, the current resolution in this study is not sufficient to account for this within-city variability and thus may overlook exposure inequalities faced by urban minorities and underestimate the true associations. Another notable limitation is that we relied on the self-report and electronic health record capture of both incident and prevalent cancers and did not distinguish between primary and secondary cancers. We report differences in the effect based on the source of cancer report. The degree of impact of multiple cancers is illustrated in Supplemental Table 1. Likewise, self-report data are not sufficiently detailed to allow for finer-grained analysis including reproductive or menopausal factors for breast cancer. We also found significant disparity by race in the self-reported survey data. For example, while Non-Hispanic Black participants comprised 18.76% of the overall sample population, they accounted for only 6.12% of self-reported cancers. Similarly, participants identifying as Hispanic/Latino comprised 18.03% of our sample, yet they accounted for only 5.82% of self-reported cancers. This disparity is consistent with our previous analysis of All of Us data and highlights the importance of continued engagement with populations historically underrepresented in biomedical research by both incentivizing and removing barriers to follow up data collection [43]. The difference in association between cancer risk and PM2.5 based on data source is clearly illustrated in our report. Furthermore, the representativeness of this work is limited given the sampling plan; as illustrated in Figs. 1 and 2, the health provider organizations that account for the greatest share of participant recruitment are generally located in metropolitan areas. Furthermore, at the current stage, the All of Us data used for this analysis are cross-sectional in nature as we relied on baseline data and limited longitudinal transfer of EHR. It is therefore difficult to establish temporality between air pollution and cancer outcomes and it is impossible to investigate cancer progression in relation to air pollution. However, reverse causation—the greatest concern in cross-sectional studies—is not likely in this study as higher cancer prevalence does not cause higher air pollution. The association between air pollution and cancer prevalence observed in this study still supports the adverse impact of air pollution on cancer outcomes. Likewise, the cross-sectional nature of the current data also presents the limitation of a lack of “latency” or “lag” of exposure. To address this limitation our analysis used the 10-year PM2.5 average from 2007 to 2016, aiming to cover the cancer progression stages before the study enrollment period. However, we understand that these efforts cannot completely offset the limitation induced by the study design. Some inverse associations observed in this study may be the consequence of this limitation.
The study has several notable strengths. While previous studies have been limited by small numbers of cancer cases, the sample size of this study, with more than 400,000 participants, entails the largest investigation of the association between air pollution and cancer to date. Second, research on the carcinogenicity of air pollution has long focused nearly exclusively on lung cancer, however outdoor air pollution might cause cancer at sites other than the lung through absorption, metabolism, and distribution of inhaled carcinogens. Other cancer types, including leukemia and breast cancer, have been also investigated in relation to air pollution. However, to our knowledge no study has simultaneously investigated as many cancer types as in this one. Third, the study design of All of Us will eventually enable researchers to analyze cancer risk longitudinally (although in this early analysis we are restricted to essentially cross-sectional data), thus providing additional opportunities to consider the role of air pollution in cancer occurrence and development. Many prior studies have only been able to use cancer mortality as the outcome, thus may underestimate the true odds for some cancers.
In summary, the All of Us Research Program presents significant opportunities to further evaluate the role of the environment and air pollution in cancer odds and outcomes. We have observed associations of PM2.5 exposure with several types of cancer and risks differing by race/ethnicity. This preliminary investigation suggests that some previous findings on cancer and PM2.5 are also observed in All of Us; for instance, our breast cancer results. Given the large and diverse All of Us study population, it may be possible to further consider the role of the environment on cancer disparities in addition to cancer risk in general. In the coming years, All of Us may confer sufficient study power to research the role of the environment in cancers that have historically been infeasible to investigate due to small sample size. This project should provide some preliminary insight and direction for future investigation.
Data availability
Data are owned by a third party, the All of Us Research Program. The data underlying this article were provided by the All of Us Research Program by permission. Data will be shared on request to the corresponding author with permission of All of Us. More information on data access can be found in the All of Us Research Hub (https://www.researchallofus.org).
References
EPA (2022) Air quality—National Summary United States Environmental Protection Agency. https://www.epa.gov/air-trends/air-quality-national-summary
Lane HM, Morello-Frosch R, Marshall JD, Apte JS (2022) Historical redlining is associated with present-day air pollution disparities in U.S. cities. Environ Sci Technol Lett 9(4):345–350
Collins TW, Grineski SE, Shaker Y, Mullen CJ (2022) Communities of color are disproportionately exposed to long-term and short-term PM2.5 in metropolitan America. Environ Res 214:114038
Bowe B, Xie Y, Yan Y, Al-Aly Z (2019) Burden of cause-specific mortality associated With PM2.5 air pollution in the United States. JAMA Netw Open 2(11):e1915834
Pye HOT, Appel KW, Seltzer KM, Ward-Caviness CK, Murphy BN (2022) Human-health impacts of controlling secondary air pollution precursors. Environ Sci Technol Lett 9(2):96–101
Pond ZA, Hernandez CS, Adams PJ, Pandis SN, Garcia GR, Robinson AL et al (2022) Cardiopulmonary mortality and fine particulate air pollution by species and source in a National U.S. Cohort. Environ Sci Technol 56(11):7214–7223
Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N et al (2018) The Lancet Commission on pollution and health. The Lancet 391(10119):462–512
Krewski D, Burnett RT, Goldberg MS, Hoover BK, Siemiatycki J, Jerrett M et al (2003) Overview of the reanalysis of the Harvard six cities study and American Cancer Society study of particulate air pollution and mortality. J Toxicol Environ Health A 66(16–19):1507–1551
Pope CA III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K et al (2002) Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287(9):1132–1141
Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M et al (2011) Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med 184(7):828–835
Pond ZA, Saha PK, Coleman CJ, Presto AA, Robinson AL, Arden Pope C III (2022) Mortality risk and long-term exposure to ultrafine particles and primary fine particle components in a National U.S. Cohort. Environ Int 167:07439
Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A (2015) The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525(7569):367–371
Krewski D, Jerrett M, Burnett RT, Ma R, Hughes E, Shi Y et al (2009) Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst 140:5–114; discussion 5-36
Turner MC, Krewski D, Pope CA 3rd, Chen Y, Gapstur SM, Thun MJ (2011) Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med 184(12):1374–1381
Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G et al (2013) Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 14(9):813–822
Hamra GB, Guha N, Cohen A, Laden F, Raaschou-Nielsen O, Samet JM et al (2014) Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect 122(9):906–911
Puett RC, Hart JE, Yanosky JD, Spiegelman D, Wang M, Fisher JA et al (2014) Particulate matter air pollution exposure, distance to road, and incident lung cancer in the nurses’ health study cohort. Environ Health Perspect 122(9):926–932
Josyula S, Lin J, Xue X, Rothman N, Lan Q, Rohan TE et al (2015) Household air pollution and cancers other than lung: a meta-analysis. Environ Health 14:24
Turner MC, Krewski D, Diver WR, Pope CA 3rd, Burnett RT, Jerrett M et al (2017) Ambient air pollution and cancer mortality in the cancer prevention study II. Environ Health Perspect 125(8):087013
Pedersen M, Stafoggia M, Weinmayr G, Andersen ZJ, Galassi C, Sommar J et al (2018) Is there an association between ambient air pollution and bladder cancer incidence? Analysis of 15 European cohorts. Eur Urol Focus 4(1):113–120
White AJ, Bradshaw PT, Hamra GB (2018) Air pollution and breast cancer: a review. Curr Epidemiol Rep 5(2):92–100
White AJ, Keller JP, Zhao S, Carroll R, Kaufman JD, Sandler DP (2019) Air pollution, clustering of particulate matter components, and breast cancer in the sister study: a U.S.-Wide Cohort. Environ Health Perspect 127(10):107002
Andersen ZJ, Ravnskjær L, Andersen KK, Loft S, Brandt J, Becker T et al (2017) Long-term exposure to fine particulate matter and breast cancer incidence in the Danish Nurse Cohort Study. Cancer Epidemiol Biomark Prev 26(3):428–430
Poulsen AH, Sørensen M, Andersen ZJ, Ketzel M, Raaschou-Nielsen O (2016) Air pollution from traffic and risk for brain tumors: a nationwide study in Denmark. Cancer Causes Control 27(4):473–480
Niehoff NM, Gammon MD, Keil AP, Nichols HB, Engel LS, Sandler DP et al (2019) Airborne mammary carcinogens and breast cancer risk in the Sister Study. Environ Int 130:104897
Cheng I, Tseng C, Wu J, Yang J, Conroy SM, Shariff-Marco S et al (2020) Association between ambient air pollution and breast cancer risk: the multiethnic cohort study. Int J Cancer 146(3):699–711
Jørgensen JT, Johansen MS, Ravnskjær L, Andersen KK, Bräuner EV, Loft S et al (2016) Long-term exposure to ambient air pollution and incidence of brain tumours: the Danish Nurse Cohort. Neurotoxicology 55:122–130
Pedersen M, Andersen ZJ, Stafoggia M, Weinmayr G, Galassi C, Sørensen M et al (2017) Ambient air pollution and primary liver cancer incidence in four European cohorts within the ESCAPE project. Environ Res 154:226–233
Raaschou-Nielsen O, Pedersen M, Stafoggia M, Weinmayr G, Andersen ZJ, Galassi C et al (2017) Outdoor air pollution and risk for kidney parenchyma cancer in 14 European cohorts. Int J Cancer 140(7):1528–1537
Nagel G, Stafoggia M, Pedersen M, Andersen ZJ, Galassi C, Munkenast J et al (2018) Air pollution and incidence of cancers of the stomach and the upper aerodigestive tract in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Int J Cancer 143(7):1632–1643
IARC (2013) Outdoor air pollution. In: Humans IWGotEoCRt (ed). International Agency for Research on Cancer, World Health Organization, Lyon
Pritchett N, Spangler EC, Gray GM, Livinski AA, Sampson JN, Dawsey SM et al (2022) Exposure to outdoor particulate matter air pollution and risk of gastrointestinal cancers in adults: a systematic review and meta-analysis of epidemiologic evidence. Environ Health Perspect 130(3):36001
Turner MC, Andersen ZJ, Baccarelli A, Diver WR, Gapstur SM, Pope CA III et al (2020) Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J Clin 70(6):460–479
Raaschou-Nielsen O, Beelen R, Wang M, Hoek G, Andersen ZJ, Hoffmann B et al (2016) Particulate matter air pollution components and risk for lung cancer. Environ Int 87:66–73
IARC (2014) Diesel and gasoline engine exhausts and some nitroarenes. In: Humans IWGotEoCRt (ed). International Agency for Research on Cancer, World Health Organization, Lyon
IARC (2012) Arsenic, metals, fibres and dusts. In: Humans IWGotEoCRt (ed). International Agency for Research on Cancer, World Health Organization, Lyon
Denny JC, Devaney SA, Gebo KA (2019) The “All of Us” Research Program. Reply N Engl J Med 381(19):1884–1885
Collins FS, Varmus H (2015) A new initiative on precision medicine. N Engl J Med 372(9):793–795
Aldrighetti CM, Niemierko A, Van Allen E, Willers H, Kamran SC (2021) Racial and ethnic disparities among participants in precision oncology clinical studies. JAMA Netw Open 4(11):e2133205
Li J, Li X, Zhang S, Snyder M (2019) Gene-environment interaction in the era of precision medicine. Cell 177(1):38–44
McCarthy M, Birney E (2021) Personalized profiles for disease risk must capture all facets of health. Nature 597(7875):175–177
Whitsel LP, Wilbanks J, Huffman MD, Hall JL (2019) The role of government in precision medicine, precision public health and the intersection with healthy living. Prog Cardiovasc Dis 62(1):50–54
Aschebrook-Kilfoy B, Zakin P, Craver A, Shah S, Kibriya MG, Stepniak E et al (2022) An overview of cancer in the first 315,000 all of us participants. PLoS ONE 17(9):e0272522
Denny JC, Rutter JL, Goldstein DB, Philippakis A, Smoller JW, Jenkins G et al (2019) The “All of Us” Research Program. N Engl J Med 381(7):668–676
All of Us Research Hub: National Institutes of Health 2022. https://www.researchallofus.org/. Accessed 25 Oct 2022
Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J et al (2019) An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int 130:104909
Di Q, Kloog I, Koutrakis P, Lyapustin A, Wang Y, Schwartz J (2016) Assessing PM2.5 exposures with high spatiotemporal resolution across the continental United States. Environ Sci Technol 50(9):4712–4721
Di Q, Koutrakis P, Schwartz J (2016) A hybrid prediction model for PM2.5 mass and components using a chemical transport model and land use regression. Atmos Environ 131:390–399
Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH et al (2018) Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 392(10159):1923–1994
Health Effects Institute (2019) State of Global Air 2019. https://www.stateofglobalair.org
Zare Sakhvidi MJ, Lequy E, Goldberg M, Jacquemin B (2020) Air pollution exposure and bladder, kidney and urinary tract cancer risk: a systematic review. Environ Pollut 267:115328
Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G et al (2009) Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54(3):659–667
Guo L, Byun HM, Zhong J, Motta V, Barupal J, Zheng Y et al (2014) Effects of short-term exposure to inhalable particulate matter on DNA methylation of tandem repeats. Environ Mol Mutagen 55(4):322–335
Panni T, Mehta AJ, Schwartz JD, Baccarelli AA, Just AC, Wolf K et al (2016) Genome-wide analysis of DNA methylation and fine particulate matter air pollution in three study populations: KORA F3, KORA F4, and the normative aging study. Environ Health Perspect 124(7):983–990
Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M et al (2004) Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109(21):2655–2671
Hu H, Dailey AB, Kan H, Xu X (2013) The effect of atmospheric particulate matter on survival of breast cancer among US females. Breast Cancer Res Treat 139(1):217–226
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867
DeNardo DG, Coussens LM (2007) Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res 9(4):212
Baumgarten SC, Frasor J (2012) Minireview: inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol Endocrinol 26(3):360–371
Gabet S, Lemarchand C, Guénel P, Slama R (2021) Breast cancer risk in association with atmospheric pollution exposure: a meta-analysis of effect estimates followed by a health impact assessment. Environ Health Perspect 129(5):57012
DuPré NC, Hart JE, Holmes MD, Poole EM, James P, Kraft P et al (2019) Particulate matter and traffic-related exposures in relation to breast cancer survival. Cancer Epidemiol Biomark Prev 28(4):751–759
Goldberg MS, Villeneuve PJ, Crouse D, To T, Weichenthal SA, Wall C et al (2019) Associations between incident breast cancer and ambient concentrations of nitrogen dioxide from a national land use regression model in the Canadian National Breast Screening Study. Environ Int 133(Pt B):105182
Villeneuve PJ, Goldberg MS, Crouse DL, To T, Weichenthal SA, Wall C et al (2018) Residential exposure to fine particulate matter air pollution and incident breast cancer in a cohort of Canadian women. Environ Epidemiol 2(3):e021
Bai L, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A et al (2020) Exposure to ambient air pollution and the incidence of lung cancer and breast cancer in the Ontario Population Health and Environment Cohort. Int J Cancer 146(9):2450–2459
Hart JE, Bertrand KA, DuPre N, James P, Vieira VM, VoPham T et al (2018) Exposure to hazardous air pollutants and risk of incident breast cancer in the Nurses’ Health Study II. Environ Health 17(1):28
Yu Q, Zhang L, Hou K, Li J, Liu S, Huang K, Cheng Y (2021) Relationship between air pollutant exposure and gynecologic cancer risk. Int J Environ Res Public Health 18(10):5353. https://doi.org/10.3390/ijerph18105353
Jerrett M, Burnett RT, Ma R, Pope CA 3rd, Krewski D, Newbold KB et al (2005) Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology 16(6):727–736
Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL et al (2007) Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356(5):447–458
Acknowledgments
We would like to thank each and every All of Us Research Program participant, without whom this work would not be possible. We would also like to thank the All of Us frontline research staff, as well as the past and present All of Us Research Program principal investigators.
Funding
The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276. This work was also supported by the NIEHS funded Chicago Center for Health and the Environment (P30 ES027792-05A1).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data management was performed by AC. Analysis was performed by AC and overseen by JL. Maps were generated by NR. The first draft of the manuscript was written by BAK, JL, and AC and all authors reviewed and edited subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study, and the All of Us Research Program protocol was approved by the NIH All of Us Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Craver, A., Luo, J., Kibriya, M.G. et al. Air quality and cancer risk in the All of Us Research Program. Cancer Causes Control 35, 749–760 (2024). https://doi.org/10.1007/s10552-023-01823-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-023-01823-7