Abstract
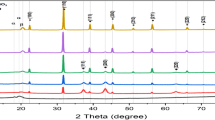
In this study, polyaniline (PANI)/SnO2, and polyvinyl-alcohol (PVA)/PANI/SnO2 nanocomposite as polymer hybrid electrodes were synthesized from tin chloride precursor and PANI/PVA polymer by sol–gel solution method. Also, for comparison, the SnO2:F (transparent conductive oxide, FTO) and aluminium (Al) on PET substrate as metallic flexible electrodes were deposited by thermal vacuum evaporation and spray pyrolysis methods. Two different molar concentrations were used to synthesize PANI/SnO2 conductive electrodes. The conductive polymer nanocomposites and polymeric electrodes were characterized by XRD analysis, FE-SEM imaging, UV–Vis and FTIR spectroscopy. In the synthesized PANI/SnO2 nanocomposite with a larger amount of SnO2 solution (15 ml), the sharp peaks of PANI are completely removed. The XRD results for FTO/PET showed that at T = 200°C, the amorphous spectrum decreased sharply due to a decrease in the PET peak. Also, the peaks of SnO2 with the tetragonal and cubic structures were observed with the preferred direction (110). FE-SEM images of FTO thin films deposited on the PET polymer substrate at T = 200°C showed that the flat plates formed by bonding of SnO2 nanoparticles formed on the polymer substrate. Also, the optical bandgap of the PANI/SnO2 nanocomposite showed that with increasing the volume of SnO2 solution, the amount of energy gap increases. The results of FTIR spectroscopy showed that with increasing SnO2, there is no difference in the type of factor group and only affects the intensity of the peaks and leads to a little shift of the peaks to the modes of pure polyaniline.











Similar content being viewed by others
References
Beygisangchin M, Abdul Rashid S, Shafie S, Sadrolhosseini A R and Lim H N 2021 Polymers 13 12
Bhadra J, Alkareem A and Al-Thani N 2020 J. Polym. Res. 27 122
Namsheer K and Chandra Sekhar Rout 2021 RSC Adv. 11 10
Najjar R, Katourani S A and Hosseini M G 2018 Prog. Org. Coat. 124 110
Lu H, Li X and Lei Q 2021 Front. Chem. 9 732132
Jeon I Y and Baek J B 2010 Materials 3 6
Bhadra S, Khastgir D, Singha N K and Lee J H 2009 Prog. Polym. Sci. 34 8
Duarte L T, e Silva E M, Branco J R and Lins V F 2004 Surf. Coat. Technol. 182 2
Sarda P, Hanan J C, Lawrence J G and Allahkarami M 2022 J. Polym. Sci. 60 1
Uranga J, Nguyen B T, Si T T, Guerrero P and de la Caba K 2020 Polymers 12 2
Khademi N, Bagheri-Mohagheghi M M and Shirpay A 2022 Opt. Quant. Electron 54 130
Shaban M, Almohammedi A, Saad R and M El Sayed A 2022 Nanomaterials 12 453
Su S J and Kuramoto N 2000 Synthet. Metal. 114 2
Mo T C, Wang H W, Chen S Y and Yeh Y C 2008 Ceram. Int. 34 7
Kondawar S B, Agrawal S P, Nimkar S H, Sharma H J and Patil P T 2012 Adv. Mater. Lett. 3 5
Khuspea G D, Navalea S T, Bandgara D K, Chougulea M A and Patil V B 2014 Electron. Mater. Lett. 1 10
Feng Q, Zhang H, Shi Y, Yu X and Lan G 2021 Polymers 13 1360
Kim H, Auyeung R C Y and Piqué A 2011 Thin Solid Films 520 1
Huang X, Yu Z, Huang Sh, Zhang Q, Li D, Luo Y et al 2010 Mater. Lett. 64 15
Sajedi S A, Bagheri-Mohagheghi M M and Shirpay A 2023 Opt. Quant. Electron. 55 1
Kondawar S B, Deshpande M D and Agrawal S P 2012 Int. J. Compos. Mater. 2 3
Sarmah S and Kumar A 2013 Bull. Mater. Sci. 36 1
Shirpay A and Bagheri Mohagheghi M M 2021 Mater. Sci. Eng.: B 272 115351
Shayeghi Sabzevar P, Bagheri-Mohagheghi M M and Shirpay A 2023 J. Mater. Sci.: Mater. Electron. 34 791
Shirpay A and Bagheri Mohagheghi M M 2022 Physica B 627 413615
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajedi, S.A., Bagheri-Mohagheghi, M.M. & Shirpay, A. PANI/SnO2 nanoparticle, FTO/PET and Al/PET as hybrid nanocomposite soft electrodes synthesized by sol–gel, spray pyrolysis and thermal vacuum evaporation methods. Bull Mater Sci 47, 9 (2024). https://doi.org/10.1007/s12034-023-03081-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-023-03081-4