Abstract
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental condition characterized by altered brain connectivity and function. In this study, we employed advanced bioinformatics and explainable AI to analyze gene expression associated with ASD, using data from five GEO datasets. Among 351 neurotypical controls and 358 individuals with autism, we identified 3,339 Differentially Expressed Genes (DEGs) with an adjusted p-value (≤ 0.05). A subsequent meta-analysis pinpointed 342 DEGs (adjusted p-value ≤ 0.001), including 19 upregulated and 10 down-regulated genes across all datasets. Shared genes, pathogenic single nucleotide polymorphisms (SNPs), chromosomal positions, and their impact on biological pathways were examined. We identified potential biomarkers (HOXB3, NR2F2, MAPK8IP3, PIGT, SEMA4D, and SSH1) through text mining, meriting further investigation. Additionally, we shed light on the roles of RPS4Y1 and KDM5D genes in neurogenesis and neurodevelopment. Our analysis detected 1,286 SNPs linked to ASD-related conditions, of which 14 high-risk SNPs were located on chromosomes 10 and X. We highlighted potential missense SNPs associated with FGFR inhibitors, suggesting that it may serve as a promising biomarker for responsiveness to targeted therapies. Our explainable AI model identified the MID2 gene as a potential ASD biomarker. This research unveils vital genes and potential biomarkers, providing a foundation for novel gene discovery in complex diseases.
Similar content being viewed by others
Introduction
Autism Spectrum disorder (ASD) is a neurodevelopmental disorder mainly affecting the brain, immune system, and gastrointestinal tract (Chow et al. 2012). Its characteristics include and are not limited to restricted interests, repetitive behaviors, and social communication disorders (Alonso-Gonzalez et al. 2018). ASD is generally considered a multifactorial disorder with genetic effects and non-genetic components of risk. The exact cause of ASD has not been fully defined, but a strong genetic component has been demonstrated through familial studies (Eissa et al. 2018). In addition, genetic studies have found that alterations within the developmental pathways of the neuronal and axonal systems appear to be strongly involved in synaptogenesis due to single-gene mutations (Eissa et al. 2018). Microarray is an important first-line technique to reveal the genetic contribution to ASD and other complicated neurobehavioral disorders (Mehta et al. 2010; Sarachana and Hu 2013; Benítez-Burraco 2020). This method has been used to study the pathology of ASD and to detect differentially expressed genes (DEGs) among individuals who are autistic and normal (Kuwano et al. 2011; Sekaran et al. 2021; Voineagu et al. 2011; Hu and Lai 2013; Sarachana and Hu 2013). Although microarray technology is a strategy for identifying associated genes and underlying biological mechanisms, genes defined in one study may not be detected in others (Zhang et al. 2017). The reliability and generalization of results can be improved by combining information from multiple reported studies and datasets (Ramasamy et al. 2008; Udhaya Kumar et al. 2021; Datta et al. 2023). The study of complicated disorders such as ASD requires a background understanding of their pathogenesis, evolutionary history, and mapping of genetic loci using an integrated analysis. Network analysis for autism-related genes through protein-protein interactions (PPIs) is an alternative method to evaluate the dynamic influences of associated candidate genes. Such an assessment can suggest a list of gene-drug targets (Corominas et al. 2014).
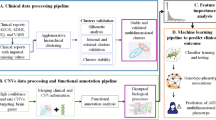
Recently, next-generation sequencing (NGS) techniques have transformed the capacity of researchers and clinicians to gather genetic data. Moreover, machine learning (ML) methods have been integrated with NGS in recent years to revolutionize bioinformatics tools and approaches. (Hassan et al. 2022). Numerous literature assesses and showcases the various applications of ML and AI in disease and drug research (Li et al. 2021). Databases dedicated to understanding the molecular genetics of diseases serve as valuable tools for investigating the epidemiology of ASD, providing comprehensive insights into the clinical manifestations and genetic backgrounds of individuals with ASD (Tye et al. 2018). To understand the genetic etiology of ASD, it is beneficial to employ an integrated, multidisciplinary approach. Modern bioinformatics techniques are instructive for deciphering ASD data. Additionally, computational research has shed light on the underlying mechanisms of ASD, confirming the importance of such tools in understanding this complex disorder (Rosenberg et al. 2015; Ray et al. 2019). Furthermore, numerous computational studies on various pathogenesis have been reported (Habib et al. 2020; Younes et al. 2020; Micheal et al. 2020). Utilizing diverse datasets and advanced bioinformatics tools, we have achieved a holistic understanding of ASD. We examined genetic variants across the different GEO datasets, patients with ASD, and normal controls and identified pathogenic SNPs linked to ASD genes. Our exploration extended to recognizing consistently associated SNPs or genes, understanding protein-protein interactions, and analyzing gene pathways and ontologies related to ASD. Additionally, we harnessed text-mining tools to gauge gene frequency in ASD literature and employed the SHAP model to uncover potential ASD biomarkers.
Materials and methods
Dataset information
The dataset used for this analysis can be accessed through the Gene Expression Omnibus database (GEO) with IDs GSE29918, GSE29691, GSE37772, GSE111175, and GSE42133 (Luo et al. 2012; Pramparo et al. 2015a, b; Gazestani et al. 2019). These data were based on multiple platforms and the same cell types, showing gene expression profiles of 709 samples (Lymphoblastoid cell lines or leukocytes) isolated from 351 normal controls and 358 autistic individuals. The detection and quantification of DEGs in the transcription profiles were evaluated using the ImaGEO tool with standard parameters and an adjusted p-value of 0.05, and the method used was Fisher (Toro-Domínguez et al. 2019).
Protein-protein interactions (PPIs), text mining, and gene ontology analysis
A PPI network assessment was performed using the STRING database (Szklarczyk et al. 2017, 2019, 2021). This analysis will show the protein interaction between the studied ones. For the enrichment analysis, there are many tools to characterize the genes’ functions. Thus, all DEGs studied were entered into DAVID, Shinygo, and GOnet tools using the Entrez Gene ID to obtain enrichment categories, GO enrichment, and the pathways (Pomaznoy et al. 2018; Ge et al. 2020; Sherman et al. 2022). The ClinVar database was used to search for known disease-associated SNPs and their risk factors, wherein the common DEGs between the five datasets served as the input (Landrum et al. 2016). To detect the positions of the pathogenic SNPs in the genes, all the DEGs studied were entered into g: Profiler, a web server for functional enrichment analysis (Raudvere et al. 2019). Following this, only SNPs in delay or autism and intellectual or neurological diseases were submitted to SNPnexus and g: Profiler to annotate SNPs and genes according to effect and biological pathways (Dayem Ullah et al. 2018).
We performed text mining to identify previously reported genes associated with ASD. Our automated extraction process sourced data from many published research related to ASD. From PubMed, 8,923 article summaries were downloaded that contained the query “autism + gene” from 2000 until May 1, 2021. In-home Python scripts were used to extract genes mentioned within the text. The complete text mining approach used for this study is shown in Fig. 1. Also, to find common genes between autism, schizophrenia, and other neural and brain disorders, we used DAVID PubMed results. For that, PubMed IDs resulting from DAVID were used to download the summaries of the articles until May 1, 2021. Then, in-house Python-based scripts were used to detect articles with autism, schizophrenia, and neural or brain, then extract genes mentioned within the text from each type of neurological disorder. The difference between the two text mining methods is that DAVID’s method searched the specific gene results in all published articles, not just in autism articles, like the first method.
ASD gene biomarker identification by explainable artificial intelligence
Explainable Artificial Intelligence (XAI) offers significant potential for interpreting intricate representations amid diverse information sources. While ML algorithms are extensively employed to analyze biological data and identify potential disease biomarkers, the inherent black-box nature of traditional ML models complicates the understanding and interpretation of their decision-making processes (Lundberg and Lee 2017). XAI algorithms address these challenges by providing better insights into the evident decisions of the predictions by the AI models, ensuring accuracy, fairness, and transparency. These explanations assist researchers and experts in understanding the base factors and technical features the models consider when identifying biomarkers (Sekaran et al. 2023). Among the five datasets used in this study, we selected GSE42133 to perform biomarker experimentation based on a few selection criteria. The sample size, the number of classes in each dataset, and the differential expression analysis results were considered for selecting the dataset. The 56 control samples were assigned a binary value of 0, and the 91 affected patients’ samples were assigned a binary value of 1.
Shapley additive explanations
SHapley Additive exPlanations (SHAP) is an XAI framework that interprets the predictions of machine learning models. It provides the contribution of every variable to the final prediction made by the model based on its importance. The method follows the cooperative game theory and the concept of Shapley values introduced by Lloyd Shapley in 1953 (Shapley 1953). SHAP describes the contribution of prediction by assigning a SHAP value to each feature of an input instance. SHAP value represents the fluctuation in the expected prediction in both cases where the particular feature is included compared to its exclusion impact (Lundberg et al. 2020). The following terms represent each parameter of the SHAP function, where X is the input features of a sample, f denotes the machine-learning model trained to predict the output, and φ is the SHAP value function.
The SHAP value function φ takes the following form:
In the above equation, φ0 represents the expected model output for a baseline reference. φi(xi) represents the contribution of feature xi to the model output. It identifies the change in the prediction when feature xi is included compared to when it is excluded, considering all possible feature subsets.
Results
Identifying the differentially expressed genes (DEGs)
Utilizing analytical samples from 351 neurotypical controls and 358 autism-affected subjects, 3,339 DEGs were identified with an adjusted p-value threshold of 0.05 of the five databases, all sourced from the same sample archetype, notably lymphoblastoid cell lines (LCL) and leukocytes. A heatmap of all DEGs was created (Fig. 2A). As shown in Fig. 2A, the heat map represents all DEGs over-expressed or under-expressed samples from each dataset. Meta-analysis results showed that 342 DGEs were found in all datasets with an adjusted p-value of 0.001, and these genes were used for the rest of the following analyses. Of them, 19 genes were upregulated, while 10 were down-regulated in all five datasets (Fig. 2B).
Aberrant expression of genes. (A) Complete heatmap of all DEGs. Two groups, ASD with purple and control with green, are discriminated clearly with down-regulated genes with green color and upregulated genes with red. (B) All common genes (up and down-regulated) between the five datasets. Up- and down-regulated genes with red and blue, respectively
PPI network analysis
PPI networks are a graphical representation of the interactions between proteins within a biological system. The PPI shown in Fig. 3 exhibits the relationship found among most of the genes (261 out of 342) in this study. Moreover, about 44% of the genes in the network (115 genes) have more than 0.9 node scores. For example, ATR, CHEK1, GUCY1A3, GUCY1B3, and MRPL34 are some of the genes with the highest node score, 0.999.
Enrichment analysis and SNP analysis
DAVID Functional Annotation, ShinyGo, and GOnet tools performed a functional enrichment analysis involving cytobands, chromosomes, diseases, and pathways for DEGs shared between the five datasets with adjusted p-value < 0.001. However, Gene Ontology (GO) enrichment was conducted for only genes involved in PPI’s interaction network. The results showed that chromosomes 19 and 17 have the highest number of genes, 31 and 30, respectively (Table 1). For cytobands, Xq13.1 and 22q13.1 have the highest numbers of genes (4 for each). Cancer (24%), pharmacogenomic (19.9%), and neurological (19.6%) were the most diseases found. Polymorphism (62.9%), alternative splicing (60.8%), and phosphoprotein (51.2%) were the highest keywords in our genes (Table 1). Also, according to DAVID-PubMed mining text results, we found 14, 16, and 211 genes related to autism, Schizophrenia, and neural or brain disorders. For the pathways, caffeine metabolism, cocaine addiction, and choline metabolism in cancer were found from the top pathways related to ASD genes, resulting in our study (Fig. 4A). The GO analysis from the biological process revealed that GO terms related to the responsibility to stimulus, signaling, and development or regulation of the nervous system have 171 (65%), 138 (53%), and 53 (20%) genes, respectively (Fig. 4B). ClinVar database was used to search for pathogenic SNPs, associated diseases, and associated risk factors (Fig. 4C). All pathogenic SNPs found, 753, 208, 100, 83, and 67, were associated with cancer, mental or intellectual, neuronal diseases, Noonan syndrome, and delay or autism, respectively (Fig. 4C). The positions of SNPs in autism and the most related diseases (delay, intellectual, and mental diseases) were detected on the GRCh38 Chromosomes (Fig. 5A). In addition, many non-synonymous variants were detected as potential biomarkers of response to targeted therapies for ASD, such as FGFR inhibitors (Fig. 5B). Also, the variant effects of the pathogenic SNPs were detected (Fig. 5C). These terms convey information about the effects each allele of the variant may have on each gene (Agrahari et al. 2018, 2019)..
Text mining
The text mining analysis revealed 3270 genes previously documented in the scientific literature discussing autism disorder-related genes. Among these genes were 50 genes common between our meta-analysis and the text mining from other articles. Moreover, 26 genes of the common 50 genes, such as DLG4 (discs large MAGUK scaffold protein 4), ATR (ATR serine/threonine kinase), and SH2B1 (SH2B adaptor protein 1), were found here in 0.9 of the PPI interaction networks. Furthermore, we tried to focus on the genes related to the development or regulation of the nervous system (53 genes), and using the results of text mining with articles published previously and PPI’s score > = 0.9, we could detect 13 genes that seem to be more involved in ASD’s disorders. These genes are DLG4, MIF, ATR, TAF1, MED12, MBP, ATF4, ITGA3, CREB1, DSC2, EFNB3, YY1, and GDI1.
Dataset selection criteria
The bioinformatics analysis is conducted on GSE29918, GSE29691, GSE37772, GSE111175, and GSE42133 to identify molecular insights about ASD. An attempt has been made to develop machine-learning models for finding the gene biomarkers from these datasets. Initially, the datasets GSE29918 and GSE29691 are ruled out for their sample size of 14 and 15, respectively. In the next step, the total number of genes from each dataset is reduced during differential expression analysis. The datasets GSE37772 and GSE111175 listed only two (RPS4Y1, KDM5D) and zero genes, respectively, based on the criteria (adj. p-value < 0.01 & logFC > 0.5 or logFC < -0.5) as differentially expressed. We exercised caution in selecting gene expression datasets to ensure they would not compromise the sensitivity of the ML model. So, the GSE37772 and GSE111175 datasets are excluded from performing machine learning analysis. GSE42133 is examined, identified as relevant, and selected to build the XAI model.
Shapley additive explanations
The initial DEGs of GSE42133 containing 172 gene biomarkers that satisfied the corresponding criteria logFC > 0.5 or logFC < -0.5 and adj. p-value < 0.01 were scrutinized with a recursive feature elimination algorithm (RFE), a wrapper-based feature selection method. It selects the most relevant features in a dataset by iteratively eliminating less important features based on a specified model’s performance. The subset generated by the algorithm is trained with support vector machines to determine the scores of each set. The resultant subset with the best performance contains 46 genes as candidate markers of ASD. This 46-feature subset is further trained in the next process using an extreme gradient boosting algorithm (XGBoost) for XAI model preparation. The trained model is fed as an input into the SHAP framework to perform the interpretation, thereby understanding the decision of every prediction made by the black-box model. The overall importance of each feature based on its contribution towards the prediction is depicted in Fig. 6A-B, containing a global bar and bee-swarm plot. The higher mean SHAP value (mean absolute value) denotes that such features significantly impact the predictions. MID2 stands on top with the highest score of + 1.21, followed by AK3 (+ 1) and RHOQ (+ 0.84) in three consecutive scores. The bee-swarm plot provides insight into the genes with positive and negative SHAP values. The positive SHAP value represents the influence of genes irrespective of the feature value (increased/decreased expression levels) to “ASD,” and the negative SHAP value denotes the “control” prediction. The data points visualized in the beeswarm plot in “cyan” show decreased expression levels, and the “violet” indicates increased expression levels for the particular gene for all the samples. In Fig. 6C-D, the parameter E[f(X)] is the baseline, and f(x) is the value predicted by our model. The values on the x-axis assigned to each gene are the actual expression values on the particular sample. In comparing the results between the two samples of ASD and control, the genes MID2 and AK3 clearly explain that the increased expression levels are influencing the predictions of ASD and the low expression levels to control. Figure 6E-F represents the summary and cohort plots generated based on the Shapley values. The cohort plot clearly shows that the increased expression levels of MID2 predict the samples as ASD, whereas the lower levels are classified into normal samples. The summary plot is the smooth version of the bee-swarm plot with a violin-like representation.
(A) Global bar plot with mean SHAP value of each gene denoting its contribution to the predictions. (B) Beeswarm plot with SHAP value (negative value represents the genes influencing the prediction towards “Control,” whereas the positive value indicates the genes predicting the sample as “ASD” under different expression levels of each gene). (C, D). A local bar plot was generated for a random control sample (left) and an ASD sample (right) taken from the dataset to interpret the prediction. The results show that the increased expression levels of MID2 (5.534) by the model are predicted as ASD, whereas the expression levels are decreased on the samples predicted as control (5.214). (E) Summary plot of top 10 genes influencing the predictions. (F) Cohort plot revealing the most influential gene predicting the samples with the highest accuracy (MID2). The values MID2 < 5.40 indicate the lower expression levels of the gene influencing the predictions of samples as control (47 out of 56) and MID2 > 5.40 as ASD (100 out of 91) with nine misclassifications
Discussion
Rapid advancements in computing have made their way into high throughput bioinformatics strategies, with AI and advanced ML models leading the charge in bioinformatics and computational biology. Enhanced data processing predictive modeling is now employed to develop accurate and precise therapeutic strategies (Subramanian et al. 2020; Bonkhoff and Grefkes 2022). This work identified DEGs across multiple GEO datasets related to ASD. Subsequent bioinformatics analyses were performed to investigate these DEGs’ putative functions and molecular interactions. Furthermore, XAI was employed to identify candidate biomarker genes for ASD. Cell type-specific gene expression profiling analyses are useful approaches to identifying genes specifically expressed in certain cell types and play an important role in ASD (Raudvere et al. 2019). However, gene expression in peripheral blood cells is very sensitive to stress so gene expression patterns may be altered during cell isolation and purification (Pascual et al. 2010). This study used five different datasets from human blood studies (Table 2). Figure 2 A illustrates comparable expression levels between control (healthy) and autistic individuals across the analyzed datasets.
Furthermore, we identified shared genes and loci, pathogenic SNPs, distributions of SNP frequencies, and the chromosomal locations of these SNPs. We also mapped the PPI network for these associated genes (Fig. 3). Through comprehensive analysis, we pinpointed the genes most closely related to ASD and related conditions. Subsequent enrichment analysis was conducted to understand the functional implications of these identified genes. Our analysis identified 19 upregulated and 10 downregulated genes (Fig. 2B). The following genes exhibited up-regulation: UTY (ubiquitously transcribed tetratricopeptide repeat-containing, Y-linked), GUCY1A3 (Guanylate cyclase soluble subunit alpha-3), NR2F2 (nuclear receptor subfamily 2 group F member 2), and GUCY1B3 (Guanylate cyclase soluble subunit beta-1).
Conversely, the genes CYP20A1 (cytochrome P450 family 20 subfamily A member 1) and CTBP1 (C-terminal binding protein 1) were significantly down-regulated. A comparison with text mining results from extant literature on ASD indicates that these genes were not previously associated with ASD. As a result, they may represent novel candidate genes potentially implicated in ASD pathogenesis.
Upon further annotation of the 342 DEGs with an adjusted p-value of 0.001, chromosomes 17 and 19 were identified to harbor the highest number of these DEGs (Table 1). Numerous cytogenetic investigations have revealed anomalies on chromosomes 17 and 19, including duplications, deletions, and inversions, within regions housing potential ASD-associated genes (Miles 2011; Butler et al. 2015). Moreover, 22q13.1 and Xq13.1 were found previously as chromosome locations for many related ASD genes (Butler et al. 2015). The q arm at position 13.1 of chromosome 22 had the highest number of ASD-associated genes compared to other locations. Also, it has been discovered that new microduplication in Xq13.1 is linked to autism and speech delay (Gumus 2019). In the current study, genes associated with oncological, pharmacogenomic, and neurological domains exhibited the highest frequency of investigation (Table 1) (Crawley et al. 2016; Xiong et al. 2019). Genes associated with cancer processes were found belong to various biological functions, including cellular proliferation (e.g., C-terminal binding protein 1 - CTBP1), cell adhesion (e.g., integrin subunit alpha 2 - ITGA2 and cadherin 1 - CDH1), growth and development (e.g., platelet-derived growth factor subunit A - PDGFA), and cell death promotion (e.g., axin 1 - AXIN1). Pharmacogenomics studies have systematically investigated the associations between genetic variants, therapeutic responses, and adverse reactions. Historically, the primary focus has been the study of antidepressants, antipsychotics, and stimulants, the predominant pharmacological classes utilized in treating ASD (Brown et al. 2017).
Our study revealed that genetic polymorphisms and alternative splicing significantly influence our datasets related to ASD. Unlike conditions such as Fragile X Syndrome, defined by specific gene mutations, ASD does not have unique polymorphisms that can act as definitive biomarkers for prediction. This complexity results in genetic variants across numerous genes associated with ASD risk. It is also worth noting that while ASD prevalence is rising, the exact rates, especially within families already affected by ASD, require further verification (Steinman 2018). In addition, evidence suggests that disruption of the normal splicing sites can lead to many human diseases, like ASD (Cieply and Carstens 2015; Quesnel-Vallières et al. 2016).
In our work, the caffeine metabolism pathway featured the most significant proportion of involved genes (Fig. 4A). Research into metabolic profiles in children with ASD has underscored the significance of caffeine metabolism as a central pathway when comparing typically healthy children with those who have ASD (Rangel-Huerta et al. 2019). For our GO analysis, we focused on the biological processes (BP) that encompass stimulus, signaling, and development or regulation of the nervous-related processes (Fig. 4B). As a result of our investigation, it became evident that most of the genes examined in our study play a significant role in biological processes related to Autism Spectrum Disorder (ASD). We sought to pinpoint common genes between two distinct groups: 19 genes that exhibited increased activity and 53 genes associated with the development or regulation of the nervous system, as revealed by our biological analysis.
We identified seven shared genes between these groups: HOXB3, NR2F2, MAPK8IP3, PIGT, SEMA4D, and SSH1. Strikingly, six of these genes, excluding PLK2, were absent from the results obtained through text mining. Therefore, these six genes represent promising candidates not previously associated with ASD and could play pivotal roles in the disorder.
We identified 1,286 pathogenic SNPs. Most of these SNPs were distributed among disorders with pathways that are either directly linked or related to ASD. Specifically, they were found in cancer (58.55%), mental or intellectual disorders (16.17%), neuronal diseases (7.78%), Noonan syndrome (6.45%), developmental delays or autism (5.21%), brain-related conditions (3.65%), and mitochondrial disorders (2.18%) (Fig. 4C). The significant representation of genes in cancer pathways corroborates a previous study that highlighted shared pathways, risk genes, and drug targets between cancer and ASD (Crawley et al. 2016). While many diseases can be categorized as mental or neuronal, there is a recognized overlap among them (Sullivan et al. 2019). This underscores the significance of our ASD-related findings and suggests that the identified SNPs should be considered strongly related to the pathogenesis of ASD.
Furthermore, 14 pathogenic SNPs were directly associated with ASD. We noticed these pathogenic SNPs were the most found in chromosome 10 and chromosome X, with 7 and 5 pathogenic SNPs, respectively. Many studies linked many genes on chromosomes X and 10 with ASD. On the other hand, we found that chromosome X has the highest number of pathogenic SNPs in our study that are related to delay or autism and intellectual or mental diseases (Fig. 5A). A recent study on analysis of the genetics related to ASD and intellectual disability found many genes on the X chromosome (Chiurazzi et al. 2020). Moreover, as it is known, the ASD ratio is higher in males than females. Therefore, many theories explain the relationships between the genes on chromosome X and the high ratio in males (Baron-Cohen et al. 2011). In addition, for chromosome 10, a study proved that some genes affect the abilities of autistic (Chapman et al. 2011).
The biomarker chart displays potential biomarkers that exhibit responsiveness to targeted therapies designed for ASD (Fig. 5B). As seen in Fig. 5B, the presence of the highest number of pathogenic SNPs in FGFR inhibitor suggests that it may serve as a promising potential biomarker for responsiveness to targeted therapies for ASD in further studies. Previous studies found that interruption of signaling of FGFR pathways could act as a possible function in ASD’s molecular pathology (Wu et al. 2016, 2020). As can be seen in Fig. 5C, the missense variants are the most frequent in ASD-diseased patients, and this is in agreement with a recent paper that sequenced a large number of autistic individuals (6430) and found the highest frequency was for missense in exons of protein-coding regions (Satterstrom et al. 2020). Another recent study on missense variants in ASD found that many missense variants in autistic individuals damage central proteins and interactions (Chen et al. 2020).
While conducting experiments using the five datasets to identify biomarkers of DEGs for the preparation of the XAI pipeline, we identified two specific genes, RPS4Y1 and KDM5D, exhibiting statistical significance for the GSE37772 according to the dataset selection criteria. The dataset GSE111175 does not show any DEGs. The RPS4Y1 and KDM5D are Y-linked chromosome genes. The RPS4Y1 regulates trophoblast cell migration and invasion through the STAT3epithelial–mesenchymal transition pathway (Chen et al. 2018), and emerging research hints at its potentially pivotal role in neurogenesis (Khani et al. 2022). The KDM5D contains coding information for a protein featuring zinc finger domains, and a small peptide derived from this protein serves as a minor histocompatibility antigen. Hatch et al. (2021) and Zamurrad et al. (2018) highlighted the significance of members within the KDM5 gene family in neurodevelopment. Several studies have explored the association of the KDM5 gene family with ASD and identified pathological significance (El Hayek et al. 2020). The RPS4Y1, the chromosome Y encoded gene and also an inhibitor of STAT3 signaling, is identified as a contributor of ASD specific to male predominance. The GSE42133 containing 172 gene biomarkers, further scrutinized into 46 with RFE, is trained with the XAI model to reveal the key marker discriminating the ASD and control samples.
The XAI model identified MID2 as a key biomarker differentiating control from ASD groups. Elevated MID2 gene expression levels have been associated with a potential predictive factor for ASD. The MID2 protein, known as Midline-1-interacting protein 2, is encoded by the MID2 gene located on the X chromosome in humans (Geetha et al. 2014). Research has unveiled the biological significance of MID2, which is associated with conditions such as intellectual disability. With a SHAP value of + 1.21, this biomarker is linked to diseases like autism affecting cognitive and motor functions. MID2 is a member of the E3 ubiquitin ligases protein family, which controls cellular activities and aids in protein breakdown. The ubiquitin-proteasome pathway, which is responsible for the selective degradation of proteins within cells, specifically involves the MID2 (Bonini et al. 2017). Mutations or abnormalities in the MID2 gene have been associated with various genetic disorders. One well-known disorder associated with MID2 is Opitz G/BBB syndrome type 1 (FG syndrome), characterized by developmental and intellectual disabilities (Ferrentino et al. 2007). Children initially diagnosed with ASD often display characteristics of FG syndrome. Exploring the protein and its associated biological pathways will provide fresh insights into the connection between the gene and ASD (Lasser et al. 2018).
Conclusion
In this study, we used comprehensive bioinformatics, advanced machine learning techniques, and XAI methodologies to unravel the complex genetic landscape of ASD. The rigorous analysis of multiple GEO datasets, alongside in-depth bioinformatics assessments, led to the identification of a significant number of DEGs that are associated with ASD. We compared our findings with similar studies to identify common trends and further elucidate certain aspects, specifically the pathogenesis and risk factors associated with SNPs. Our XAI model identified MID2 as a potential clinical biomarker for ASD. It is important to note that our analysis had limitations stemming from the unavailability of detailed clinical data, which limited the potential genotype-phenotype correlation. In the future, the analysis of multimodal genetic datasets of many patients integrated with clinical information promises to unlock profound insights into the molecular and clinical pathogenesis of ASD. This will provide a comprehensive understanding of gene functionality, gene loci, observed SNPs, dysregulated pathways in ASD, and their impact on clinical measures. Ultimately, these insights will facilitate the development of more accurate treatment approaches for ASD.
References
Agrahari AK, Kumar A, R S, et al (2018) Substitution impact of highly conserved arginine residue at position 75 in GJB1 gene in association with X-linked Charcot-Marie-tooth Disease: a computational study. J Theor Biol 437:305–317. https://doi.org/10.1016/j.jtbi.2017.10.028
Agrahari AK, Doss GPC, Siva R et al (2019) Molecular insights of the G2019S substitution in LRRK2 kinase domain associated with Parkinson’s Disease: a molecular dynamics simulation approach. J Theor Biol 469:163–171. https://doi.org/10.1016/j.jtbi.2019.03.003
Alonso-Gonzalez A, Rodriguez-Fontenla C, Carracedo A (2018) De novo mutations (DNMs) in Autism Spectrum disorder (ASD): pathway and network analysis. Front Genet 9
Baron-Cohen S, Lombardo MV, Auyeung B et al (2011) Why are Autism Spectrum conditions more prevalent in males? PLOS Biol 9:e1001081. https://doi.org/10.1371/journal.pbio.1001081
Benítez-Burraco A (2020) Genes dysregulated in the blood of people with Williams syndrome are enriched in protein-coding genes positively selected in humans. Eur J Med Genet 63:103828. https://doi.org/10.1016/j.ejmg.2019.103828
Bonini SA, Mastinu A, Ferrari-Toninelli G, Memo M (2017) Potential role of Microtubule stabilizing agents in Neurodevelopmental disorders. Int J Mol Sci 18. https://doi.org/10.3390/ijms18081627
Bonkhoff AK, Grefkes C (2022) Precision medicine in Stroke: towards personalized outcome predictions using artificial intelligence. Brain J Neurol 145:457–475. https://doi.org/10.1093/brain/awab439
Brown JT, Eum S, Cook EH, Bishop JR (2017) Pharmacogenomics of autism spectrum disorder. Pharmacogenomics 18:403–414. https://doi.org/10.2217/pgs-2016-0167
Butler MG, Rafi SK, Manzardo AM (2015) High-resolution chromosome ideogram representation of currently recognized genes for Autism Spectrum disorders. Int J Mol Sci 16:6464–6495. https://doi.org/10.3390/ijms16036464
Chapman NH, Estes A, Munson J et al (2011) Genome-scan for IQ discrepancy in autism: evidence for loci on chromosomes 10 and 16. Hum Genet 129:59–70. https://doi.org/10.1007/s00439-010-0899-z
Chen X, Tong C, Li H, Peng W, Li R, Luo X, Ge H, Ran Y, Li Q, Liu Y, Xiong X (2018) Dysregulated expression of RPS4Y1 (ribosomal protein S4, Y-linked 1) impairs STAT3 (signal transducer and activator of transcription 3) signaling to suppress trophoblast cell migration and invasion in preeclampsia. Hypertens Dallas Tex 71:481–490. https://doi.org/10.1161/HYPERTENSIONAHA.117.10250
Chen S, Wang J, Cicek E et al (2020) De novo missense variants disrupting protein-protein interactions affect risk for autism through gene co-expression and protein networks in neuronal cell types. Mol Autism 11:76. https://doi.org/10.1186/s13229-020-00386-7
Chiurazzi P, Kiani AK, Miertus J et al (2020) Genetic analysis of intellectual disability and autism. Acta Bio Medica Atenei Parm 91:e2020003. https://doi.org/10.23750/abm.v91i13-S.10684
Chow ML, Pramparo T, Winn ME et al (2012) Age-Dependent Brain Gene expression and copy number anomalies in Autism Suggest distinct pathological processes at Young Versus mature ages. PLOS Genet 8:e1002592. https://doi.org/10.1371/journal.pgen.1002592
Cieply B, Carstens RP (2015) Functional roles of alternative splicing factors in human Disease. Wiley Interdiscip Rev RNA 6:311–326. https://doi.org/10.1002/wrna.1276
Corominas R, Yang X, Lin GN et al (2014) Protein interaction network of alternatively spliced isoforms from brain links genetic risk factors for autism. Nat Commun 5:3650. https://doi.org/10.1038/ncomms4650
Crawley JN, Heyer W-D, LaSalle JM (2016) Autism and Cancer share risk genes, pathways, and drug targets. Trends Genet 32:139–146. https://doi.org/10.1016/j.tig.2016.01.001
Datta A, Udhaya Kumar S, D’costa M et al (2023) Identification of dysregulated canonical pathways associated with pathogenesis and progression of amyotrophic lateral Sclerosis-An integrated bioinformatics approach. Adv Protein Chem Struct Biol 134:21–52. https://doi.org/10.1016/bs.apcsb.2022.11.014
Dayem Ullah AZ, Oscanoa J, Wang J et al (2018) SNPnexus: assessing the functional relevance of genetic variation to facilitate the promise of precision medicine. Nucleic Acids Res 46:W109–W113. https://doi.org/10.1093/nar/gky399
Eissa N, Al-Houqani M, Sadeq A et al (2018) Current Enlightenment about etiology and pharmacological treatment of Autism Spectrum Disorder. Front Neurosci 12:304. https://doi.org/10.3389/fnins.2018.00304
El Hayek L, Tuncay IO, Nijem N, Russell J, Ludwig S, Kaur K, Li X, Anderton P, Tang M, Gerard A, Heinze A (2020) KDM5A mutations identified in autism spectrum disorder using forward genetics. eLife 9:e56883. https://doi.org/10.7554/eLife.56883
Ferrentino R, Bassi MT, Chitayat D et al (2007) MID1 mutation screening in a large cohort of Opitz G/BBB syndrome patients: twenty-nine novel mutations identified. Hum Mutat 28:206–207. https://doi.org/10.1002/humu.9480
Gazestani VH, Pramparo T, Nalabolu S et al (2019) A perturbed gene network containing PI3K-AKT, RAS-ERK and WNT-β-catenin pathways in leukocytes is linked to ASD genetics and symptom severity. Nat Neurosci 22:1624–1634. https://doi.org/10.1038/s41593-019-0489-x
Ge SX, Jung D, Yao R (2020) ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36:2628–2629. https://doi.org/10.1093/bioinformatics/btz931
Geetha TS, Michealraj KA, Kabra M et al (2014) Targeted deep resequencing identifies MID2 mutation for X-linked intellectual disability with varied Disease severity in a large kindred from India. Hum Mutat 35:41–44. https://doi.org/10.1002/humu.22453
Gumus E (2019) A hemizygous 370 kilobase microduplication at Xq13.1 in a three-year-old boy with autism and Speech Delay. Fetal Pediatr Pathol 38:239–244. https://doi.org/10.1080/15513815.2019.1571132
Habib PT, Alsamman AM, Hassanein SE, Hamwieh A (2020) Developing convolutional neural networks-based System for Predicting Pneumonia using X-Radiography image. Highlights Biosci 3:1–3. https://doi.org/10.36462/H.BioSci.20201
Hassan M, Awan FM, Naz A et al (2022) Innovations in Genomics and Big Data Analytics for Personalized Medicine and Health Care: a review. Int J Mol Sci 23:4645. https://doi.org/10.3390/ijms23094645
Hatch HA, Belalcazar HM, Marshall OJ, Secombe J (2021) A KDM5-Prospero transcriptional axis functions during early neurodevelopment to regulate mushroom body formation. eLife 10:e63886. https://doi.org/10.7554/eLife.63886
Hu VW, Lai Y (2013) Developing a predictive gene classifier for Autism Spectrum disorders based upon Differential Gene expression profiles of phenotypic subgroups. North Am J Med Sci 6. https://doi.org/10.7156/najms.2013.0603107
Khani F, Nafian S, Mollamohammadi S, Nemati S, Shokoohian B, Hassani SN, Baharvand H, Soleimanpour-Lichaei HR, Salekdeh GH (2022) Y chromosome genes may play roles in the development of neural rosettes from human embryonic stem cells. Stem Cell Rev Rep 18:3008–3020. https://doi.org/10.1007/s12015-022-10392-2
Kuwano Y, Kamio Y, Kawai T et al (2011) Autism-associated gene expression in peripheral leucocytes commonly observed between subjects with autism and healthy women having autistic children. PLoS ONE 6:e24723. https://doi.org/10.1371/journal.pone.0024723
Landrum MJ, Lee JM, Benson M et al (2016) ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res 44:D862–868. https://doi.org/10.1093/nar/gkv1222
Lasser M, Tiber J, Lowery LA (2018) The role of the Microtubule Cytoskeleton in Neurodevelopmental disorders. Front Cell Neurosci 12:165. https://doi.org/10.3389/fncel.2018.00165
Li Z, Jiang X, Wang Y, Kim Y (2021) Applied machine learning in Alzheimer’s Disease research: omics, imaging, and clinical data. Emerg Top Life Sci 5:765–777. https://doi.org/10.1042/ETLS20210249
Lundberg SM, Lee S-I (2017) A Unified Approach to Interpreting Model Predictions. ArXiv
Lundberg SM, Erion G, Chen H et al (2020) From local explanations to Global understanding with explainable AI for trees. Nat Mach Intell 2:56–67. https://doi.org/10.1038/s42256-019-0138-9
Luo R, Sanders SJ, Tian Y et al (2012) Genome-wide transcriptome profiling reveals the functional impact of rare de novo and recurrent CNVs in autism spectrum disorders. Am J Hum Genet 91:38–55. https://doi.org/10.1016/j.ajhg.2012.05.011
Mehta D, Menke A, Binder EB (2010) Gene expression studies in major depression. Curr Psychiatry Rep 12:135–144. https://doi.org/10.1007/s11920-010-0100-3
Micheal AO, Catherine OA, Adenike AK et al (2020) Predictive comparative antibiotic resistance (AMR) profiles of rhizobacteria genes using CARD: a bioinformatics approach. Highlights Biosci 3. https://doi.org/10.36462/H.BioSci.20223
Miles JH (2011) Autism spectrum disorders–a genetics review. Genet Med off J Am Coll Med Genet 13:278–294. https://doi.org/10.1097/GIM.0b013e3181ff67ba
Pascual V, Chaussabel D, Banchereau J (2010) A genomic approach to human autoimmune Diseases. Annu Rev Immunol 28:535–571. https://doi.org/10.1146/annurev-immunol-030409-101221
Pomaznoy M, Ha B, Peters B (2018) GOnet: a tool for interactive gene ontology analysis. BMC Bioinformatics 19:470. https://doi.org/10.1186/s12859-018-2533-3
Pramparo T, Lombardo MV, Campbell K et al (2015a) Cell cycle networks link gene expression dysregulation, mutation, and brain maldevelopment in autistic toddlers. Mol Syst Biol 11:841. https://doi.org/10.15252/msb.20156108
Pramparo T, Pierce K, Lombardo MV et al (2015b) Prediction of autism by translation and immune/inflammation coexpressed genes in toddlers from pediatric community practices. JAMA Psychiatry 72:386–394. https://doi.org/10.1001/jamapsychiatry.2014.3008
Quesnel-Vallières M, Dargaei Z, Irimia M et al (2016) Misregulation of an activity-dependent Splicing Network as a common mechanism underlying Autism Spectrum disorders. Mol Cell 64:1023–1034. https://doi.org/10.1016/j.molcel.2016.11.033
Ramasamy A, Mondry A, Holmes CC, Altman DG (2008) Key issues in conducting a meta-analysis of gene expression microarray datasets. PLoS Med 5:e184. https://doi.org/10.1371/journal.pmed.0050184
Rangel-Huerta OD, Gomez-Fernández A, de la Torre-Aguilar MJ et al (2019) Metabolic profiling in children with autism spectrum disorder with and without mental regression: preliminary results from a cross-sectional case-control study. Metabolomics off J Metabolomic Soc 15:99. https://doi.org/10.1007/s11306-019-1562-x
Raudvere U, Kolberg L, Kuzmin I et al (2019) G:profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 47:W191–W198. https://doi.org/10.1093/nar/gkz369
Ray M, Mishra J, Priyadarshini A, Sahoo S (2019) In silico identification of potential drug target and analysis of effective single nucleotide polymorphisms for autism spectrum disorder. Gene Rep 16:100420. https://doi.org/10.1016/j.genrep.2019.100420
Rosenberg A, Patterson JS, Angelaki DE (2015) A computational perspective on autism. Proc Natl Acad Sci U S A 112:9158–9165. https://doi.org/10.1073/pnas.1510583112
Sarachana T, Hu VW (2013) Genome-wide identification of transcriptional targets of RORA reveals direct regulation of multiple genes associated with autism spectrum disorder. Mol Autism 4:14. https://doi.org/10.1186/2040-2392-4-14
Satterstrom FK, Kosmicki JA, Wang J et al (2020) Large-scale exome sequencing study implicates both developmental and functional changes in the Neurobiology of Autism. Cell 180:568–584e23. https://doi.org/10.1016/j.cell.2019.12.036
Sekaran K, Sudha M (2021) Predicting autism spectrum disorder from associative genetic markers of phenotypic groups using machine learning. J Ambient Intell Humaniz Comput 12(3):3257–3270. https://doi.org/10.1007/s12652-020-02155-z
Sekaran K, Alsamman AM, George Priya Doss C, Zayed H (2023) Bioinformatics investigation on blood-based gene expressions of Alzheimer’s Disease revealed ORAI2 gene biomarker susceptibility: an explainable artificial intelligence-based approach. Metab Brain Dis 38:1297–1310. https://doi.org/10.1007/s11011-023-01171-0
Shapley LS (1953) Stochastic games. Proc Natl Acad Sci U S A 39:1095–1100. https://doi.org/10.1073/pnas.39.10.1095
Sherman B, Hao M, Leidos L et al (2022) DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res 50. https://doi.org/10.1093/nar/gkac194
Steinman G (2018) GENE POLYMORPHISM IN THE GENESIS OF AUTISM
Subramanian M, Wojtusciszyn A, Favre L et al (2020) Precision medicine in the era of artificial intelligence: implications in chronic Disease management. J Transl Med 18:472. https://doi.org/10.1186/s12967-020-02658-5
Sullivan JM, De Rubeis S, Schaefer A (2019) Convergence of spectrums: neuronal gene network states in autism spectrum disorder. Curr Opin Neurobiol 59:102–111. https://doi.org/10.1016/j.conb.2019.04.011
Szklarczyk D, Morris JH, Cook H et al (2017) The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res 45:D362–D368. https://doi.org/10.1093/nar/gkw937
Szklarczyk D, Gable AL, Lyon D et al (2019) STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47:D607–D613. https://doi.org/10.1093/nar/gky1131
Szklarczyk D, Gable AL, Nastou KC et al (2021) The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 49:D605–D612. https://doi.org/10.1093/nar/gkaa1074
Toro-Domínguez D, Martorell-Marugán J, López-Domínguez R et al (2019) ImaGEO: integrative gene expression meta-analysis from GEO database. Bioinformatics 35:880–882. https://doi.org/10.1093/bioinformatics/bty721
Tye C, Runicles AK, Whitehouse AJO, Alvares GA (2018) Characterizing the interplay between Autism Spectrum Disorder and Comorbid Medical conditions: an integrative review. Front Psychiatry 9:751. https://doi.org/10.3389/fpsyt.2018.00751
Udhaya Kumar S, Saleem A, Thirumal Kumar D, Anu Preethi V, Younes S, Zayed H, Tayubi IA (2021) George Priya Doss C. A systemic approach to explore the mechanisms of drug resistance and altered signaling cascades in extensively drug-resistant Tuberculosis. Adv Protein Chem Struct Biol 127:343–364. https://doi.org/10.1016/bs.apcsb.2021.02.002
Voineagu I, Wang X, Johnston P et al (2011) Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474:380–384. https://doi.org/10.1038/nature10110
Wu YE, Parikshak NN, Belgard TG, Geschwind DH (2016) Genome-wide, integrative analysis implicates microRNA dysregulation in autism spectrum disorder. Nat Neurosci 19:1463–1476. https://doi.org/10.1038/nn.4373
Wu X, Li W, Zheng Y (2020) Recent progress on relevant microRNAs in Autism Spectrum disorders. Int J Mol Sci 21:5904. https://doi.org/10.3390/ijms21165904
Xiong J, Chen S, Pang N et al (2019) Neurological Diseases with Autism Spectrum Disorder: role of ASD risk genes. Front Neurosci 13:349. https://doi.org/10.3389/fnins.2019.00349
Younes S, Shi Z, Zayed H (2020) Genetic variations associated with coronary artery Disease and Myocardial Infarction in the arab world: a systematic review and meta-analysis. Highlights Biosci 3. https://doi.org/10.36462/H.BioSci.20213
Zamurrad S, Hatch HAM, Drelon C, et al (2018) A Drosophila model of intellectual disability caused by mutations in the histone demethylase KDM5. Cell Rep 22:2359–2369. https://doi.org/10.1016/j.celrep.2018.02.018
Zhang S, Deng L, Jia Q et al (2017) dbMDEGA: a database for meta-analysis of differentially expressed genes in autism spectrum disorder. BMC Bioinformatics 18:494. https://doi.org/10.1186/s12859-017-1915-2
Acknowledgements
The authors would like to take this opportunity to thank the management of Vellore Institute of Technology (VIT), Vellore, Tamil Nadu, India, for providing the necessary facilities and encouragement to carry out this work. The authors would also like to acknowledge the efforts of the personnel at Qatar University, Doha, Qatar.
Funding
Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
The study design and data collection involved LDN, AMA, MHA, NAD, KS, SK, KV, and GPDC. LDN, AD, AMA, MHA, NAD, KS, SK, KV, GPDC, and HZ acquired, analyzed, interpreted the results, and drafted the manuscript. GPDC and HZ supervised the entire study. All authors edited and approved the submitted version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nahas, L.D., Datta, A., Alsamman, A.M. et al. Genomic insights and advanced machine learning: characterizing autism spectrum disorder biomarkers and genetic interactions. Metab Brain Dis 39, 29–42 (2024). https://doi.org/10.1007/s11011-023-01322-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-023-01322-3