Abstract
Background
Myocardial ischemia/reperfusion (I/R) injury is closely related with cardiovascular diseases; however, the underlying pathogenic mechanisms remain not fully understood. This study sought to investigate the effect and mechanisms of PIM3 implicated in myocardial I/R injury using a rat model of myocardial I/R injury and a cell model of oxygen–glucose deprivation/reoxygenation (OGD/R) induction.
Methods
The morphology changes were detected by HE staining while cell viability was accessed by the CCK-8 method. The characteristics of ferroptosis were evaluated by ROS production, MDA content, SOD level, iron content, TfR1, FTH1, and GPX4 expression.
Results
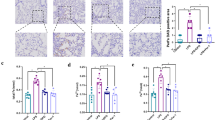
Myocardial I/R operation increased myocardial tissue damage in rats, while OGD/R treatment reduced the viability of H9c2 cells. Both myocardial I/R operation and OGD/R stimulation increased ferroptosis, as demonstrated by elevated ROS, MDA, iron content, decreased SOD level, upregulation of TfR1, and downregulation of FTH1 and GPX4. Additionally, myocardial I/R modeling or OGD/R treatment enhanced the expression of PIM3. Silencing of PIM3 inhibited ferroptosis, which resulted in alleviated myocardial I/R-induced damage and improved H9c2 cell survival.
Conclusions
Our findings highlight a vital role of PIM3 in myocardial I/R injury, indicating that PIM3-targeting ferroptosis may be a promising target for the development of novel therapies of myocardial I/R injury-associated diseases.





Similar content being viewed by others
Data availability
The data are available from the corresponding author on reasonable request.
References
Amaral EP, Costa DL, Namasivayam S, Riteau N, Kamenyeva O, Mittereder L, Mayer-Barber KD, Andrade BB, Sher A (2019) A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J Exp Med 216:556–570
Bi X, Zhang G, Wang X, Nguyen C, May HI, Li X, Al-Hashimi AA, Austin RC, Gillette TG, Fu G, Wang ZV, Hill JA (2018) Endoplasmic reticulum chaperone GRP78 Protects heart from Ischemia/reperfusion injury through Akt activation. Circ Res 122:1545–1554
Du J, Zheng X, Cai S, Zhu Z, Tan J, Hu B, Huang Z, Jiao H (2015) MicroRNA506 participates in pancreatic cancer pathogenesis by targeting PIM3. Mol Med Rep 12:5121–5126
Fang Y, Chen X, Tan Q, Zhou H, Xu J, Gu Q (2021) Inhibiting ferroptosis through disrupting the NCOA4-FTH1 interaction: a new mechanism of action. ACS Cent Sci 7:980–989
Feng H, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka JM, Upadhyayula PS, Canoll P, Uchida K, Soni RK, Hadian K, Stockwell BR (2020) Transferrin receptor is a specific ferroptosis marker. Cell Rep 30(3411–3423):e3417
Gao, Y., Xu, W., Guo, C., and Huang, T. (2022) GATA1 regulates the microRNA3283p/PIM1 axis via circular RNA ITGB1 to promote renal ischemia/reperfusion injury in HK2 cells. International journal of molecular medicine 50
Kong N, Chen X, Feng J, Duan T, Liu S, Sun X, Chen P, Pan T, Yan L, Jin T, Xiang Y, Gao Q, Wen C, Ma W, Liu W, Zhang M, Yang Z, Wang W, Zhang R, Chen B, Xie T, Sui X, Tao W (2021) Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharmaceutica Sinica B 11:4045–4054
Li W, Li W, Leng Y, Xiong Y, Xia Z (2020) Ferroptosis Is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol 39:210–225
Li X, Ma N, Xu J, Zhang Y, Yang P, Su X, Xing Y, An N, Yang F, Zhang G, Zhang L, Xing Y (2021) Targeting ferroptosis: pathological mechanism and treatment of ischemia-reperfusion injury. Oxid Med Cell Longev 2021:1587922
Liang D, Minikes AM, Jiang X (2022) Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell 82:2215–2227
Liao YH, Xia N, Zhou SF, Tang TT, Yan XX, Lv BJ, Nie SF, Wang J, Iwakura Y, Xiao H, Yuan J, Jevallee H, Wei F, Shi GP, Cheng X (2012) Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol 59:420–429
Liu, S., Yan, S., Zhu, J., Lu, R., Kang, C., Tang, K., Zeng, J., Ding, M., Guo, Z., Lai, X., Jiang, Y., Wu, S., Zhou, L., Sun, L., and Zhou, Z. W. (2022) Combination RSL3 Treatment Sensitizes Ferroptosis- and EGFR-Inhibition-Resistant HNSCCs to Cetuximab. International journal of molecular sciences 23
Ljungman PLS, Andersson N, Stockfelt L, Andersson EM, Nilsson Sommar J, Eneroth K, Gidhagen L, Johansson C, Lager A, Leander K, Molnar P, Pedersen NL, Rizzuto D, Rosengren A, Segersson D, Wennberg P, Barregard L, Forsberg B, Sallsten G, Bellander T, Pershagen G (2019) Long-term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ Health Perspect 127:107012
Luo M, Wu L, Zhang K, Wang H, Zhang T, Gutierrez L, O’Connell D, Zhang P, Li Y, Gao T, Ren W, Yang Y (2018) miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ 25:1457–1472
Mikkers H, Nawijn M, Allen J, Brouwers C, Verhoeven E, Jonkers J, Berns A (2004) Mice deficient for all PIM kinases display reduced body size and impaired responses to hematopoietic growth factors. Mol Cell Biol 24:6104–6115
Miyamoto HD, Ikeda M, Ide T, Tadokoro T, Furusawa S, Abe K, Ishimaru K, Enzan N, Sada M, Yamamoto T, Matsushima S, Koumura T, Yamada KI, Imai H, Tsutsui H (2022) Iron overload via heme degradation in the endoplasmic reticulum triggers ferroptosis in myocardial ischemia-reperfusion injury. JACC Basic Translat Sci 7:800–819
Mukaida N, Wang YY, Li YY (2011) Roles of Pim-3, a novel survival kinase, in tumorigenesis. Cancer Sci 102:1437–1442
Mung KL, Eccleshall WB, Santio NM, Rivero-Muller A, Koskinen PJ (2021) PIM kinases inhibit AMPK activation and promote tumorigenicity by phosphorylating LKB1. Cell Commun Signal 19:68
Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA (2007) Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med 13:1467–1475
Orellana DI, Santambrogio P, Rubio A, Yekhlef L, Cancellieri C, Dusi S, Giannelli SG, Venco P, Mazzara PG, Cozzi A, Ferrari M, Garavaglia B, Taverna S, Tiranti V, Broccoli V, Levi S (2016) Coenzyme a corrects pathological defects in human neurons of PANK2-associated neurodegeneration. EMBO Mol Med 8:1197–1211
Qi Q, Pan Y, Han S, Liao H, Jiang Y, Shen J, Zhong L, Wang X, Chen J (2019) PIM3 functions as oncogenic factor and promotes the tumor growth and metastasis in colorectal cancer. Anat Rec 302:1552–1560
Ren C, Yang T, Qiao P, Wang L, Han X, Lv S, Sun Y, Liu Z, Du Y, Yu Z (2018) PIM2 interacts with tristetraprolin and promotes breast cancer tumorigenesis. Mol Oncol 12:690–704
Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, Brill A, Wang Y, Wagner DD (2014) VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 123:141–148
Severino, P., D'Amato, A., Pucci, M., Infusino, F., Adamo, F., Birtolo, L. I., Netti, L., Montefusco, G., Chimenti, C., Lavalle, C., Maestrini, V., Mancone, M., Chilian, W. M., and Fedele, F. (2020) Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. International journal of molecular sciences 21
Shan X, Lv ZY, Yin MJ, Chen J, Wang J, Wu QN (2021) The protective effect of cyanidin-3-glucoside on myocardial ischemia-reperfusion injury through Ferroptosis. Oxid Med Cell Longev 2021:8880141
Wang G, Liu G, Ye Y, Fu Y, Zhang X (2019) Bufothionine exerts anti-cancer activities in gastric cancer through Pim3. Life Sci 232:116615
Xu S, Wu B, Zhong B, Lin L, Ding Y, Jin X, Huang Z, Lin M, Wu H, Xu D (2021) Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2) /System xc-/ glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 12:10924–10934
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156:317–331
Yang H, He K, Dong W, Fang J, Zhong S, Tang L, Long L (2020) PIM-1 may function as an oncogene in cervical cancer via activating the EGFR signaling. Int J Biol Markers 35:67–73
Yuan Y, Wang C, Zhuang X, Lin S, Luo M, Deng W, Zhou J, Liu L, Mao L, Peng W, Chen J, Wang Q, Shu Y, Xue Y, Huang P (2022) PIM1 promotes hepatic conversion by suppressing reprogramming-induced ferroptosis and cell cycle arrest. Nat Commun 13:5237
Zhang X, Song M, Kundu JK, Lee MH, Liu ZZ (2018) PIM Kinase as an executional target in cancer. J Cancer Prevention 23:109–116
Zhao WK, Zhou Y, Xu TT, Wu Q (2021) Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury. Oxid Med Cell Longev 2021:9929687
Zhao L, Wang Y, Min X, Yang H, Zhang P, and Zeng Q, (2010) Ischemia-reperfusion injury up-regulates Pim-3 gene expression in myocardial tissue. Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban 30, 704-708
Funding
This research was supported by the Hunan Provincial Health and Family Planning Commission Project (20180200), Hunan Provincial Science and Technology Department Project (2020SK51824), Hunan Provincial Education Department Project (21B0416), Hunan Provincial Health Commission (B202301047951).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Ting Li and Yushan Yang; (II) Administrative support: Xuefeng Xu; (III) Collection and assembly of data: Fangyao Liu and Ying Tan; (IV) Data analysis and interpretation: Yutao Peng (V) Manuscript writing: All authors; The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Informed consent
Not Applicable.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Animal Care Committee of University of South China.
Consent to publish
All authors agree to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, T., Liu, F., Tan, Y. et al. PIM3 regulates myocardial ischemia/reperfusion injury via ferroptosis. Genes Genom 46, 161–170 (2024). https://doi.org/10.1007/s13258-023-01475-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-023-01475-6