Abstract
Background
Anti-disialoganglioside (anti-GD2) monoclonal antibodies are effective immunotherapeutic drugs for treating neuroblastoma, yet their toxicity spectrum is unclear.
Objective
This study aimed to assess the toxicity profiles of three anti-GD2 monoclonal antibodies (dinutuximab, dinutuximab β, and naxitamab) in clinical applications by mining and evaluating the adverse drug reaction (ADR) signals from the US Food and Drug Administration Adverse Event Reporting System.
Methods
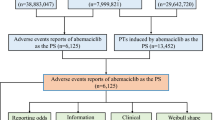
Data in the US Food and Drug Administration Adverse Event Reporting System from the time anti-GD2 monoclonal antibodies became available in the market to the first quarter of 2023 were searched. The signals of anti-GD2 monoclonal antibody-associated ADRs were quantified using four types of algorithms, including the reporting odds ratio, the proportional reporting ratio, the combination of the proportional reporting ratio and χ2 statistic method used by the UK Medicines and Healthcare Products Regulatory Agency, and the Bayesian confidence propagation neural network. The ADRs were categorized by System Organ Class based on the Medical Dictionary for Regulatory Activities, and were sorted according to the frequency and signal strength of ADRs.
Results
A total of 370 adverse drug event reports with anti-GD2 monoclonal antibodies listed as the ‘primary suspected drugs’ were identified, with 116 ADR signals detected, of which 22 were not in the drug labels. Among the adverse drug event reports, 276 reports concerned dinutuximab/dinutuximab β as primary suspected drugs with 90 ADR signals, involving 19 System Organ Classes, of which 21 signals were not in the label; 94 adverse drug event reports concerned naxitamab as the primary suspected drug with 26 ADR signals, involving 11 System Organ Classes, of which one was not in the label. For dinutuximab/dinutuximab β-related ADRs, the top five most frequent were “fever”, “abdominal pain”, “elevated aspartate aminotransferase (AST)”, “elevated alanine aminotransferase (ALT)” and “hypotension”; the top five most intensive signals were “hypoalbuminemia”, “elevated AST”, “capillary leakage syndrome”, “hypoxia” and “elevated ALT”. For naxitamab-related ADRs, the top five most frequent were “hypotension”, “pain”, “urticarial”, “hypertension” and “rash”; the top five most intensive signals were “hypotension”, “urticaria”, “hypoxemia”, “bronchospasm” and “hypertension”. Involved System Organ Classes included “investigations” and “respiratory, thoracic and mediastinal disorders” containing the most types of ADR signals in dinutuximab/dintuximab β-related ADRs and naxitamab-related ADRs, respectively.
Conclusions
Our study comprehensively analyzed the toxicity profiles of anti-GD2 monoclonal antibodies and provides an important reference for clinical monitoring and ADR identification of these drugs.

Similar content being viewed by others
References
Jin QY, Du SB, Yuan XJ. Exploring the prognosis of neuroblastoma in adolescents and adults: a case series and literature review. Neoplasma. 2022;69(2):464–73.
Spasov N, Spasova M. Early use of dinutuximab beta in patients with high-risk neuroblastoma. Case Rep Pediatr. 2021;2021:6610955.
Cabral J, Fernandez EI, Toy B, Secola R. Multidisciplinary clinical care in the management of patients receiving anti-GD2 immunotherapy for high-risk neuroblastoma. Paediatr Drugs. 2023;25(1):13–25.
Mastrangelo S, Rivetti S, Triarico S, Romano A, Attinà G, Maurizi P, et al. Mechanisms, characteristics, and treatment of neuropathic pain and peripheral neuropathy associated with dinutuximab in neuroblastoma patients. Int J Mol Sci. 2021;22(23):12648.
Ahmed AA, Zhang L, Reddivalla N, Hetherington M. Neuroblastoma in children: update on clinicopathologic and genetic prognostic factors. Pediatr Hematol Oncol. 2017;34(3):165–85.
Chan GC, Chan CM. Anti-GD2 directed immunotherapy for high-risk and metastatic neuroblastoma. Biomolecules. 2022;12(3):358.
Furman WL. Monoclonal antibody therapies for high risk neuroblastoma. Biologics. 2021;15:205–19.
Larrosa C, Mora J, Cheung NK. Global impact of monoclonal antibodies (mAbs) in children: a focus on anti-GD2. Cancers. 2023;15(14):3729.
Dalianis T, Lukoseviciute M, Holzhauser S, Kostopoulou ON. New approaches towards targeted therapy for childhood neuroblastoma. Anticancer Res. 2023;43(9):3829–39.
Cheung IY, Cheung NV, Modak S, Mauguen A, Feng Y, Basu E, et al. Survival impact of anti-GD2 antibody response in a phase II ganglioside vaccine trial among patients with high-risk neuroblastoma with prior disease rogression. J Clin Oncol. 2021;39(3):215–26.
Yu AL, Gilman AL, Ozkaynak MF, Naranjo A, Diccianni MB, Gan J, et al. Long-term follow-up of a phase III study of ch14.18 (dinutuximab) + cytokine immunotherapy in children with high-risk neuroblastoma: COG Study ANBL0032. Clin Cancer Res. 2021;27(8):2179–89.
Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30(26):3264–70.
Flaadt T, Ebinger M, Schreiber M, Ladenstein RL, Simon T, Lode HN, et al. Multimodal therapy with consolidating haploidentical stem cell transplantation and dinutuximab beta for patients with high-risk neuroblastoma and central nervous system relapse. J Clin Med. 2023;12(19):6196.
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34.
Perez Horta Z, Goldberg JL, Sondel PM. Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy. Immunotherapy. 2016;8(9):1097–117.
Ladenstein R, Pötschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1617–29.
Kushner BH, Cheung IY, Modak S, Basu EM, Roberts SS, Cheung NK. Humanized 3F8 anti-GD2 monoclonal antibody dosing with granulocyte-nacrophage colony-stimulating factor in patients with resistant neuroblastoma:a phase 1 clinical trial. JAMA Oncol. 2018;4(12):1729–35.
Achbergerová M, Hederová S, Mikesková M, Husáková K, Hrašková A, Kolenová A. Implementation of immunotherapy into the treatment of neuroblastoma-single center experience with the administration of dinutuximab and management of its adverse effects. Klin Onkol. 2020;33(5):372–8.
Muñoz JP, Larrosa C, Chamorro S, Perez-Jaume S, Simao M, Sanchez-Sierra N, et al. Early salvage chemo-immunotherapy with irinotecan, temozolomide and naxitamab plus GM-CSF (HITS) for patients with primary refractory high-risk neuroblastoma provide the best chance for long-term outcomes. Cancers. 2023;15(19):4837.
Varo A, Castañeda A, Chamorro S, Muñoz JP, Gorostegui M, Celma MS, et al. Novel infusion strategy reduces severe adverse events caused by the anti-GD2 monoclonal antibody naxitamab. Front Oncol. 2023;13:1164949.
Kushner BH, Modak S, Kramer K, Basu EM, Iglesias-Cardenas F, Roberts SS, et al. Immunotherapy with anti-G(D2) monoclonal antibody in infants with high-risk neuroblastoma. Int J Cancer. 2023;152(2):259–66.
Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2013;10(7):796–803.
Nazha B, Inal C, Owonikoko TK. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol. 2020;10:1000.
Markham A. Naxitamab: first approval. Drugs. 2021;81(2):291–6.
Ladenstein R, Weixler S, Baykan B, Bleeke M, Kunert R, Katinger D, et al. Ch14.18 antibody produced in CHO cells in relapsed or refractory Stage 4 neuroblastoma patients: a SIOPEN phase 1 study. MAbs. 2013;5(5):801–9.
Chen JJ, Huo XC, Wang SX, Wang F, Zhao Q. Data mining for adverse drug reaction signals of daptomycin based on real-world data: a disproportionality analysis of the US Food and Drug Administration Adverse Event Reporting System. Int J Clin Pharm. 2022;44(6):1351–60.
van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10.
Shao QH, Yin XD, Zeng N, Zhou ZX, Mao XY, Zhu Y, et al. Stevens-Johnson syndrome following non-steroidal anti-inflammatory drugs: a real-world analysis of post-marketing surveillance data. Front Pediatr. 2022;10: 896867.
Yang H, Yu X, An Z. Cutaneous toxicity associated with enfortumab vedotin: a real-world study leveraging U.S. Food and Drug Administration Adverse Event Reporting System. Front Oncol. 2021;11:801199.
Guan Y, Ji L, Zheng L, Yang J, Qin Y, Ding N, et al. Development of a drug risk analysis and assessment system and its application in signal excavation and analysis of 263 cases of fluoroquinolone-induced adverse reactions. Front Pharmacol. 2022;13: 892503.
Voeller J, Sondel PM. Advances in anti-GD2 immunotherapy for treatment of high-risk neuroblastoma. J Pediatr Hematol Oncol. 2019;41(3):163–9.
Dhillon S. Dinutuximab: first global approval. Drugs. 2015;75(8):923–7.
Zeng Y, Fest S, Kunert R, Katinger H, Pistoia V, Michon J, et al. Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol Immunol. 2005;42(11):1311–9.
Machy P, Mortier E, Birklé S. Biology of GD2 ganglioside: implications for cancer immunotherapy. Front Pharmacol. 2023;14:1249929.
Chung C, Boterberg T, Lucas J, Panoff J, Valteau-Couanet D, Hero B, et al. Neuroblastoma. Pediatr Blood Cancer. 2021;68 Suppl. 2(Suppl. 2):e28473.
Kumar H, Gupta R. Anti-disialoganglioside-2 monoclonal antibodies as an emerging therapeutic approach in treatment of high-risk neuroblastoma. Curr Pharmacol Rep. 2022;8(2):112–20.
Mueller I, Ehlert K, Endres S, Pill L, Siebert N, Kietz S, et al. Tolerability, response and outcome of high-risk neuroblastoma patients treated with long-term infusion of anti-GD(2) antibody ch1418/CHO. MAbs. 2018;10(1):55–61.
Nysom K, Morad AG, Rafael MS, Zier J, Marachelian A, Watt T, et al. Pain mitigation and management strategies for anti-GD2 infusions: an expert consensus. Pediatr Blood Cancer. 2023;70(5): e30217.
Acknowledgments
The authors thank Qiaofeng Ye from the Pennsylvania State University for improving the language of this paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by grants from the Shanghai Medicine and Health Development Foundation (No. 20221128), Shanghai Young Pharmaceutical Talent Ability Improvement Program (No. HYHZ(2023)04), and the Excellent Young Clinical Pharmacist Training Program in Children’s Hospital of Fudan University.
Conflicts of Interest/Competing Interests
Guangfei Wang, Jinglin Wang, Ruxiang Du, Yi Wang, and Zhiping Li have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Code Availability
Not applicable.
Authors’ Contributions
GFW designed the study, analyzed the data, drafted the initial manuscript, and revised the manuscript. RXD and JLW analyzed the data and searched the literature. JLW and ZPL revised the manuscript. YW designed the study and revised the manuscript. ZPL and GFW acquired the funding. All authors agreed on the journal to which the article was submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G., Wang, J., Du, R. et al. Toxicity Spectrum of Anti-GD2 Immunotherapy: A Real-World Study Leveraging the US Food and Drug Administration Adverse Event Reporting System. Pediatr Drugs 26, 175–185 (2024). https://doi.org/10.1007/s40272-023-00613-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00613-7