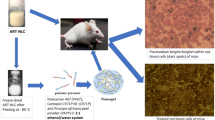
Abstract
Purpose
Methotrexate (MTX) is an antineoplastic drug used in the treatment of rheumatoid arthritis (RA). Given that it is a class IV drug with low permeability and solubility, this study aims to improve MTX skin permeation by loading it in transethosomes (TEs) and casting a transethosomal patch that allows for dose quantification to mitigate toxicity.
Methods
To accomplish this goal, MTX transethosomes (TEs) were developed using the thin film hydration technique and optimized using the Box-Behnken design (BBD) with soya phosphatidylcholine 50, Tween 80, and ethanol as independent variables using the desirability function. Furthermore, zeta potential (ZP) analysis and high-resolution transmission electron microscopy (HR-TEM) were used to confirm the stability and surface morphology of TEs. A transdermal patch was also designed and evaluated from the optimized TE (OPTZ TEs) batch using a solvent casting method with hydroxypropyl methylcellulose (HPMC) as the polymer, dimethyl sulfoxide (DMSO) as a permeation enhancer, and polyethylene glycol (PEG 400) as the plasticizer. Furthermore, ex vivo skin permeation and deposition through rat skin proved that the TE patch had better drug permeation and retention within the skin layers.
Results
The highest desirability batch had 92.19 ± 3.826 nm vesicle size, 0.35 ± 0.062 PDI, 74.05 ± 5.157% EE and 62.75 ± 4.448% Q8h which were within the predicted results. Furthermore, ZP was found to be more than − 30 mV, and HR-TEM results proved that the TE vesicles were spherical. The results of the evaluation parameters such as weight variation, folding endurance, and thickness were 0.07 ± 0.01 g, 82.3 ± 1.52 folds, and 0.93 ± 0.01, respectively, and were well within the limits. The TE patch incorporated more than 90% of the drug confirmed by the drug content analysis which allowed ex vivo permeation for almost 24 h providing a sustained release action with a permeation flux of 19 ± 1.08 and an enhancement ratio of 3.68 when compared to the MTX solution.
Conclusion
This study suggests that MTX-loaded transethosomal patch not only enhanced the skin permeation but also provided a 24-h release profile and reduced its toxicity.
Graphical Abstract









Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Abbreviations
- MTX:
-
Methotrexate
- RA:
-
Rheumatoid arthritis
- HLA:
-
Human leucocyte antigen
- TDDS:
-
Transdermal delivery system
- TEs:
-
Transethosome
- SPC 50:
-
Soya phosphatidylcholine 50
- BBD:
-
Box-Behnken design
- FTIR:
-
Fourier transform infrared spectroscopy
- ZP:
-
Zeta potential
- % EE:
-
% Entrapment efficiency
- PDI:
-
Polydispersity index
References
Jeong WY, Kwon M, Choi HE, Kim KS. Recent advances in transdermal drug delivery systems: a review. Biomater Res. 2021. https://doi.org/10.1186/s40824-021-00226-6.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008. https://doi.org/10.1038/nbt.1504.
Prajapati ST, Patel CG, Patel CN. Formulation and evaluation of transdermal patch of repaglinide. ISRN Pharm. 2011;2011: 651909. https://doi.org/10.5402/2011/651909.
Akhtar N, Singh V, Yusuf M, Khan RA. Non-invasive drug delivery technology: development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed Tech. 2020;65:243–72. https://doi.org/10.1515/bmt-2019-0019.
Tomoda K, Makino K. Nanoparticles for transdermal drug delivery system (TDDS). In: Ohshima H, Makino K editors: Colloid and interface science in pharmaceutical research and development. Elsevier BV. 2014;131–47. https://doi.org/10.1016/B978-0-444-62614-1.00007-7.
Cevc G, Vierl U. Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J Control Release. 2010. https://doi.org/10.1016/j.jconrel.2009.10.016.
Gondkar SB, Patil NR, Saudagar RB. Formulation development and characterization of drug loaded transethosomes for transdermal delivery: review article. Int J Chem Tech Res. 2017;10(6):534–44.
Honeywell-Nguyen PL, Bouwstra JA. Vesicles as a tool for transdermal and dermal delivery. Drug Discov Today Technol. 2005. https://doi.org/10.1016/j.ddtec.2005.05.003.
Bajaj KJ, Parab BS, Shidhaye SS. Nano-transethosomes: a novel tool for drug delivery through skin. Indian J Pharm Educ Res. 2021. https://doi.org/10.5530/ijper.55.1s.33.
Kumar L, Verma S, Singh K, Prasad DN, Jain AK. Ethanol based vesicular carriers in transdermal drug delivery: nanoethosomes and transethosomes in focus. NanoWorld J. 2016; https://doi.org/10.17756/nwj.2016-030.
Chacko IA, Ghate VM, Dsouza L, Lewis SA. Lipid vesicles: a versatile drug delivery platform for dermal and transdermal applications. Colloids Surfaces B Biointerfaces. 2020. https://doi.org/10.1016/j.colsurfb.2020.111262.
Fang Q, Zhou C, Nandakumar KS. Review article molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediators Inflamm. 2020; https://doi.org/10.1155/2020/3830212
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018. https://doi.org/10.1038/s41413-018-0016-9.
Anita C, Munira M, Mural Q, Shaily L. Topical nanocarriers for management of rheumatoid arthritis: a review. Biomed Pharm. 2021. https://doi.org/10.1016/j.biopha.2021.111880.
Venuturupalli S. Immune mechanisms and novel targets in rheumatoid arthritis. Immunol allergy Clin North America. 2017; https://doi.org/10.1016/j.iac.2017.01.002.
Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene – environment interaction between smoking and shared epitope genes in HLA – DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004. https://doi.org/10.1002/art.20553.
Alfredsson L, Klareskog L, Padyukov L. Gene – environment interaction between the DRB1 shared epitope and smoking in the risk of anti – citrullinated protein antibody – positive rheumatoid arthritis all alleles are important. Arthritis Rheum. 2009. https://doi.org/10.1002/art.24572.
Yap HY, Tee SZY, Wong MMT, Chow SK, Peh SC, Teow SY. Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells. 2018. https://doi.org/10.3390/cells7100161.
Alunno A, Carubbi F, Giacomelli R, Gerli R. Cytokines in the pathogenesis of rheumatoid arthritis: new players and therapeutic targets. BMC Rheumatology. 2017. https://doi.org/10.1186/s41927-017-0001-8.
Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology. 2004. https://doi.org/10.1093/rheumatology/keh201.
Saxena A, Raychaudhuri SK, Raychaudhuri SP. Rheumatoid arthritis : disease pathophysiology. Inflamm Adv Age Nutr. 2014:215–29. https://doi.org/10.1016/B978-0-12-397803-5.00018-6.
Kumar V, Kanwar JR, Verma AK. Rheumatoid arthritis: basic pathophysiology and role of chitosan nanoparticles in therapy. Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents. 2020. https://doi.org/10.1016/b978-0-12-819666-3.00016-x.
Chuang SY, Lin CH, Huang TH, Fang JY. Lipid-based nanoparticles as a potential delivery approach in the treatment of rheumatoid arthritis. Nanomaterials. 2018. https://doi.org/10.3390/nano8010042.
Law ST, Taylor PC. Role of boilogical agents in treatment of rheumatoid arthritis. Pharmacol Res. 2019. https://doi.org/10.1016/j.phrs.2019.104497.
Janakiraman K, Krishnaswami V, Sethuraman V, Rajendran V, Kandasamy R. Development of methotrexate-loaded cubosomes with improved skin permeation for the topical treatment of rheumatoid arthritis. Appl Nanosci. 2019. https://doi.org/10.1007/s13204-019-00976-9.
Jadhav P, Bothiraja C, Pawar A. Methotrexate-loaded nanomixed micelles: formulation, characterization, bioavailability, safety, and in vitro anticancer study. J Pharm Innov. 2018. https://doi.org/10.1007/s12247-018-9314-4.
Bianchi G, Caporali R, Todoerti M, Mattana P. Methotrexate and rheumatoid arthritis: current evidence regarding subcutaneous versus oral routes of administration. Adv Ther. 2016. https://doi.org/10.1007/s12325-016-0295-8.
Noack M, Miossec P. Effects of methotrexate alone or combined with arthritis-related biotherapies in an in vitro co-culture model with immune cells and synoviocytes. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.02992.
Ghosh S, Mukherjee B, Chaudhuri S, Roy T, Mukherjee A, Sengupta S. Methotrexate aspasomes against rheumatoid arthritis: optimized hydrogel loaded liposomal formulation with in vivo evaluation in Wistar rats. AAPS PharmSciTech. 2018. https://doi.org/10.1208/s12249-017-0939-2.
Demirbolat GM, Aktas E, Coskun GP, Erdogan O, Cevik O. New approach to formulate methotrexate-loaded niosomes: in vitro characterization and cellular effectiveness. J Pharm Innov. 2021. https://doi.org/10.1007/s12247-021-09539-4.
Gadad AP, Patil AS, Singh Y, Dandagi PM, Bolmal UB, Basu A. Development and evaluation of flurbiprofen loaded transethosomes to improve transdermal delivery. Indian J Pharm Educ Res. 2020. https://doi.org/10.5530/ijper.54.4.189.
Albash R, Abdelbary AA, Refai H, El-Nabarawi MA. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: in vitro, ex vivo, and in vivo evaluation. Int J Nanomed. 2019. https://doi.org/10.2147/IJN.S196771.
Rajnani N, Kurup DNS. Method development of methotrexate in phosphate buffer solution by UV-visible spectroscopy. Int J Trend Sci Res Dev. 2018; https://doi.org/10.31142/ijtsrd14256.
Rahangdale M, Pandey P. Development and characterization of apremilast transethosomal gel for transdermal delivery. Int J Pharm Sci Nanotechnol. 2021; https://doi.org/10.37285/ijpsn.2021.14.3.8.
Agrawal M, Saraf S, Pradhan M, Patel RJ, Singhvi G, Ajazuddin, et al. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed Pharmacother. 2021; https://doi.org/10.1016/j.biopha.2021.111919.
Gorle AP, Pawara IT, Achaliya AP. Design development and evaluation of transdermal drug delivery system of antipyretic agent. Int J Pharm Res Health Sci. 2017; https://doi.org/10.21276/ijprhs.2017.04.05.
Garg V, Singh H, Bhatia A, Raza K, Singh SK, Singh B, et al. Systematic development of transethosomal gel system of piroxicam: formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS PharmSciTech. 2017. https://doi.org/10.1208/s12249-016-0489-z.
Desai GN, Dandagi PM, Kazi TM. Nanosized intranasal delivery of novel self-assembled cubic liquid crystals: formulation and evaluation. J Pharm Innov. 2022. https://doi.org/10.1007/s12247-022-09695-1.
El-Sonbaty MM, Akl MA, El-Say KM, Kassem AA. Does the technical methodology influence the quality attributes and the potential of skin permeation of luliconazole loaded transethosomes?. J Drug Deliv Sci Technol. 2022. https://doi.org/10.1016/j.jddst.2022.103096.
Al-Mahallawi AM, Abdelbary AA, Aburahma MH. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int J Pharm. 2015. https://doi.org/10.1016/j.ijpharm.2015.03.033.
Moolakkadath T, Aqil M, Ahad A, Imam SS, Iqbal B, Sultana Y, et al. Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif CellsNanomed Biotechnol. 2018; https://doi.org/10.1080/21691401.2018.1469025.
Sudhakar K, Mishra V, Jain S, Rompicherla NC, Malviya N, Tambuwala MM. Development and evaluation of the effect of ethanol and surfactant in vesicular carriers on lamivudine permeation through the skin. Int J Pharm. 2021. https://doi.org/10.1016/j.ijpharm.2021.121226.
Kunieda H, Ohyama K ichi. Three-phase behavior and HLB numbers of bile salts and lecithin in a water-oil system. J Coll Interf Sci. 1990; https://doi.org/10.1016/0021-9797(90)90390-A.
Aboud HM, Ali AA, El-Menshawe SF, Elbary AA. Nanotransfersomes of carvedilol for intranasal delivery: formulation, characterization and in vivo evaluation. Drug Deliv. 2016. https://doi.org/10.3109/10717544.2015.1013587.
Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes - a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003. https://doi.org/10.1081/DDC-120025458.
Mahmoud DB, ElMeshad AN, Fadel M, Tawfik A, Ramez SA. Photodynamic therapy fortified with topical oleyl alcohol-based transethosomal 8-methoxypsoralen for ameliorating vitiligo: Optimization and clinical study. Int J Pharm. 2022. https://doi.org/10.1016/j.ijpharm.2022.121459.
Abdulbaqi IM, Darwis Y, Assi RA, Khan NAK. Transethosomal gels as carriers for the transdermal delivery of colchicine: statistical optimization, characterization, and ex vivo evaluation. Drug Design Dev Ther. 2018; https://doi.org/10.2147/DDDT.S158018.
Garg BJ, Garg NK, Beg S, Singh B, Katare OP. Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: formulation optimization, in vitro evaluation and preclinical assessment. J Drug Target. 2016. https://doi.org/10.3109/1061186X.2015.1070855.
Dhanka M, Shetty C, Srivastava R. Methotrexate loaded alginate microparticles and effect of Ca2+ post-crosslinking: an in vitro physicochemical and biological evaluation. Int J Biol Macromol. 2018. https://doi.org/10.1016/j.ijbiomac.2017.10.148.
Brito Raj S, Chandrasekhar KB, Reddy KB. Formulation, in-vitro and in-vivo pharmacokinetic evaluation of simvastatin nanostructured lipid carrier loaded transdermal drug delivery system. Future J Pharm Sci. 2019. https://doi.org/10.1186/s43094-019-0008-7.
Rashid SA, Bashir S, Naseem F, Farid A, Rather IA, Hakeem KR. Olive oil based methotrexate loaded topical nanoemulsion gel for the treatment of imiquimod induced psoriasis-like skin inflammation in an animal model. Biology. 2021. https://doi.org/10.3390/biology10111121.
Notman R, Den Otter WK, Noro MG, Briels WJ, Anwar J. The permeability enhancing mechanism of DMSO in ceramide bilayers simulated by molecular dynamics. Biophys J. 2007. https://doi.org/10.1529/biophysj.107.104703.
Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M. Enhanced transdermal delivery of an anti-hypertensive agent via nanoethosomes: statistical optimization, characterization and pharmacokinetic assessment. Int J Pharm. 2013. https://doi.org/10.1016/j.ijpharm.2013.01.011.
Kirjavainen M, Mönkkönen J, Saukkosaari M, Valjakka-Koskela R, Kiesvaara J, Urtti A. Phospholipids affect stratum corneum lipid bilayer fluidity and drug partitioning into the bilayers. J Control Release. 1999. https://doi.org/10.1016/S0168-3659(98)00152-7.
Song CK, Balakrishnan P, Shim CK, Chung SJ, Chong S, Kim DD. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids Surfaces B Biointerfaces. 2012. https://doi.org/10.1016/j.colsurfb.2011.12.004.
Raza K, Katare OP, Setia A, Bhatia A, Singh B. Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J Microencapsul. 2013. https://doi.org/10.3109/02652048.2012.717115.
Acknowledgements
We gratefully acknowledge Neon Pharmaceuticals Mumbai and Lipidome Lifesciences, Ahmedabad for providing gift samples of MTX and SPC 50, respectively. We would also like to acknowledge IIT Bombay for performing the HR-TEM analysis of the sample along with Dr. Prabhakar Kore Basic Science Research Center (BSRC) and Department of Pharmaceutical Quality Assurance, KLE College of Pharmacy, KLE Academy of Higher Education and Research (KAHER), and Belagavi for providing the facility to perform the research study. We would also like to extend our acknowledgment to Mr. Mote G.D from Annasaheb Dange College of Pharmacy, and Ashta for helping in FTIR and particle size analysis along with Dr. U.B. Bolmal from Rani Channamma University, for his invaluable guidance and help in major aspects of this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PJV: conceptualization, visualization, data curation, formal analysis, investigation, writing-original draft. VM: supervision, guidance, mentoring, and review.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Veer, P.J., Mastiholimath, V.S. Formulation, Characterization, and Optimization of Transethosomes for Enhanced Transdermal Delivery of Methotrexate. J Pharm Innov 18, 2385–2401 (2023). https://doi.org/10.1007/s12247-023-09799-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09799-2