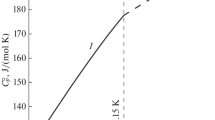
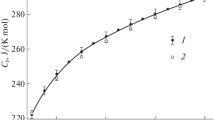
Abstract
A composition–property relationship has been developed that makes it possible to select the optimal values of the standard entropy of alkali metal borates, for which available experimental and reference data vary in wide ranges. This relationship allows one to estimate the standard entropy of unstudied alkali metal borates with sufficient validity. To ensure the reliability of the relationship, a critical analysis of the initial data borrowed from reference books and original experimental works has been carried out. Experimental measurements of low-temperature heat capacity were processed to verify the reliability of the standard entropy values of alkali metal borates presented in the literature.




Similar content being viewed by others
REFERENCES
A. A. Tupitsyn, V. A. Bychinskii, M. V. Shtenberg, et al., Russ. J. Inorg. Chem. (2023). https://doi.org/10.1134/S0036023622700243
W. M. Latimer, The Oxidation States of the Elements and Their Potentials in Aqueous Solution (Prentice-Hall Inc., New York, 1938).
H. D. B. Jenkins, J. Chem. Thermodyn. 144, 106052 (2020). https://doi.org/10.1016/j.jct.2020.106052
A. A. Tupitsyn, S. V. Yas’ko, V. A. Bychinskii, et al., Russ. J. Inorg. Chem. (2023). https://doi.org/10.1134/S0036023623600363
H. D. B. Jenkins, J. Chem. Thermodyn. 135, 278 (2019). https://doi.org/10.1016/j.jct.2019.03.013
O. V. Eremin and E. S. Epova, O. S. Rusal’, et al., Russ. J. Inorg. Chem. 61, 1003 (2016). https://doi.org/10.1134/S0036023616080064
M. K. Aldabergenov and G. T. Balakaeva, Zh. Fiz. Khim. 67, 425 (1993).
O. N. Koroleva, V. A. Bychinskii, A. A. Tupitsyn, et al., Russ. J. Inorg. Chem. 60, 1104 (2015). https://doi.org/10.1134/S0036023615090107
M. V. Shtenberg, V. A. Bychinskii, O. N. Koroleva, et al., Russ. J. Inorg. Chem. 62, 1464 (2017). https://doi.org/10.1134/S0036023617110183
L. V. Gurvich, I. V. Veits, V. A. Medvedev, et al., Thermodynamic Properties of Individual Substances, Ed. by V. P. Glushko (Nauka, Moscow, 1982) [in Russian].
V. A. Medvedev, G. A. Bergman, V. P. Vasil’ev, et al., Thermal Constants of Substances, ed. by V. P. Glushko, 10 iss. (VINITI, Moscow, 1981) [in Russian].
M. W. Chase, C. A. Davies, J. R. Downey, et al., JANAF Thermochemical Tables, 3rd ed. (Am. Inst. Phys. for the Nat. Bureau of Standards, Am. Chem. Soc., Washington D.C., New York, 1985).
D. R. Stull, D. L. Hildenbrand, F. L. Oetting, and G. C. Sinke, J. Chem. Eng. Data 15, 52 (1970). https://doi.org/10.1021/je60044a035
G. Grenier and E. F. Westrum, J. Am. Chem. Soc. 78, 6226 (1956). https://doi.org/10.1021/ja01605a004
E. F. Westrum and G. Grenier, J. Am. Chem. Soc. 79, 1799 (1957). https://doi.org/10.1021/ja01565a007
L. M. Khriplovich, A. P. Popov, and I. E. Paukov, Zh. Fiz. Khim. 50, 567 (1976).
I. E. Paukov, L. M. Khriplovich, and A. P. Popov, Zh. Fiz. Khim. 44, 547 (1970).
I. E. Paukov, L. M. Khriplovich, and A. P. Popov, Zh. Fiz. Khim. 45, 1295 (1971).
N. P. Tekhanovich, A. U. Sheleg, and Ya. V. Burak, Fiz. Tverd. Tela 32, 2513 (1990).
A. E. Aliev, V. F. Krivorotov, and P. K. Khabibullaev, Fiz. Tverd. Tela 39, 1548 (1997).
A. U. Sheleg, T. I. Dekola, N. P. Tekhanovich, and A. M. Luginets, Fiz. Tverd. Tela 39, 624 (1997).
D. D. Wagman, W. H. Evans, V. B. Parker, et al., Selected Values of Chemical Thermodynamic Properties. Compounds of Uranium, Protactinium, Thorium, Actinium, and the Alkali Metals, NBS Tech. Note 270-8 (Washington, DC, 1981).
H. Yokokawa, J. Nat. Chem. Lab. Industry 83, 27 (1988).
O. Knacke, O. Kubaschewski, and K. Hesselmann, Thermochemical Properties of Inorganic Substances (Springer-Verlag, Berlin, 1991).
O. Kubaschewski, C. B. Alock, and P. J. Spencer, Material Thermochemistry (Pergamon Press, New York, 1993).
L. B. Pankratz, Thermodynamic Properties of Carbides, Nitrides, and Other Selected Substances (U.S. Dep. of the Interior, Bureau of Mines, Washington, DC, 1994).
I. Barin, Thermochemical Data of Pure Substances (VCH-Verlag, Weinheim, 1885).
V. A. Turdakin and V. V. Tarasov, Zh. Fiz. Khim. 42, 2787 (1968).
G. S. Mel’nikov and V. V. Tarasov, Tr. Mosk. Khim.-Tekhnol. Ins. im. D. I. Mendeleeva XLI, 8 (1963).
V. A. Bychinskii, A. A. Tupitsyn, A. V. Mukhetdinova, et al., Russ. J. Inorg. Chem. 58, 1511 (2013). https://doi.org/10.1134/S0036023613120061
N. P. Tekhanovich and A. U. Sheleg, Fiz. Tverd. Tela 33, 900 (1991).
V. A. Ryabin, M. A. Ostroumov, and T. F. Svit, Thermodynamic Properties of Substances. Directory (Khimiya, Leningrad, 1977) [in Russian].
L. B. Pankratz and M. J. Ferrante, Thermodynamic Properties of For Crystalline Sodium Borates (U. S. Dept. of the Interior, Bureau of Mines, Washington, DC, 1971).
K. K. Kelley and E. G. King, Contributions to the Data on Theoretical Metallurgy, 14. Entropies of the Elements and Inorganic Compounds (U.S. Govt. Print. Off., Bureau of Mines, Washington, DC, 1961).
Funding
The study was supported by the Russian Science Foundation (project no. 22-17-20005), https://rscf.ru/project/22-17-20005/. Review and critical analysis of the data was carried out with the support of the Ministry of Science and Higher Education of the Russian Federation (State assignment no. 075-00880-22 PR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Translated by G. Kirakosyan
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tupitsin, A.A., Yas’ko, S.V., Bychinskii, V.A. et al. Evaluation of the Standard Entropy of Crystalline Alkali Metal Borates. Russ. J. Inorg. Chem. 68, 1782–1788 (2023). https://doi.org/10.1134/S0036023623602349
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023623602349