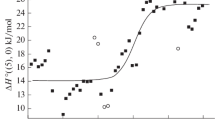
Abstract
The phase diagram of the SrF2–MgF2 system has been studied by differential thermal analysis (DTA) and X-ray powder diffraction (XRD). The stability area of the compound SrMgF4 is very small, and its extent is about 20°С between 870 and 890°С. The formation of compounds by magnesium fluoride and strontium fluoride with other metal fluorides is considered in the M versus X coordinates, where M is the cumulative moment of the cation and X is the cation electronegativity. Two areas of compound formation were identified for each of the fluorides. Magnesium and strontium fluorides are amphoteric compounds in terms of the Lewis acid and base theory.






Similar content being viewed by others
REFERENCES
E. Banks, S. Nakajima, and M. Shone, J. Electrochem. Soc. 127, 2234 (1980).
Q. Bingyi and E. Banks, Mater. Res. Bull. 17, 1185 (1982).
N. Ishizawa, K. Suda, B. E. Etschmann, et al., Acta Crystallogr., Sect. C 57, 784 (2001).
S. C. Abrahams, Acta Crystallogr., Sect. B 58, 34 (2002).
S. V. Mel’nikova, L. I. Isaenko, A. A. Goloshumova, and S. I. Lobanov, Phys. Solid State 56, 757 (2014). https://doi.org/10.1134/S1063783414040192
A. P. Pivovarova, V. A. Saltykova, O. V. Mel’nikova, and E. G. Semin, Proceedings of the VII All-Union Symposium on the Chemistry of Inorganic Fluorides, Dushanbe, 1984, p. 266 (Nauka, Moscow, 1984).
P. P. Fedorov and L. V. Medvedeva, Russ. J. Inorg. Chem. 34, 1528 (1989).
L. A. Olkhovaya, P. P. Fedorov, D. D. Ikrami, and B. P. Sobolev, J. Therm. Anal. 15, 355 (1979). https://doi.org/10.1007/BF01903660
V. A. Stasyuk, Cand. Sci. (Chem.) Dissertation, MITKhT im. M.V. Lomonosova, Moscow, 1998.
D. D. Ikrami, A. A. Luginina, L. A. Ol’khovaya, and E. D. Ruchkin, Zh. Neorg. Khim. 32, 1453 (1987).
H. G. Schnering and P. Bleckmann, Naturwissenschaften 55, 342 (1968).
P. P. Fedorov, A. A. Luginina, N. Yu. Tabachkova, A. A. Alexandrov, L. V. Bad’yanova, and S. V. Kuznetsov, Russ. J. Inorg. Chem. 67, 1211 (2022). https://doi.org/10.1134/S0036023622080101
P. P. Fedorov, A. A. Alexandrov, S. L. Korableva, and E. V. Chernova, Cryst. Res. Techn. 58, 2200251 (2023). https://doi.org/10.1002/crat.202200251
A. A. Goloshumova, L. I. Isaenko, D. Yu. Naumov, et al., J. Nanoelectron. Optoelectron. 9, 1 (2014).
I. N. Ogorodnikov, V. A. Pustovarov, L. I. Isaenko, and S. I. Lobanov, Opt. Mater. 118, 111234 (2021). https://doi.org/10.1016/j.optmat.2021.111234
I. Prigozhin and R. Defei, Chemical Thermodynamics (Nauka, Novosibirsk, 1966) [in Russian].
R. I. Efremova and E. V. Matizen, Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. 2, 3 (1970).
B. F. Naylor, J. Am. Chem. Soc. 67, 150 (1945).
P. P. Fedorov and P. I. Fedorov, Zh. Neorg. Khim. 18, 205 (1973).
P. I. Fedorov and P. P. Fedorov, Zh. Neorg. Khim. 19, 215 (1974).
P. P. Fedorov and P. I. Fedorov, Zh. Neorg. Khim. 20, 1088 (1975).
I. I. Buchinskaya and P. P. Fedorov, Russ. Chem. Rev. 73, 371 (2004). https://doi.org/10.1070/RC2004v073n04ABEH000811
R. V. Davidovich, P. P. Fedorov, and A. I. Popov, Rev. Inorg. Chem. 36, 105 (2016). https://doi.org/10.1515/revic-2015-0019
R. V. Davidovich, P. P. Fedorov, and A. I. Popov, Rev. Inorg. Chem. 37, 147 (2017). https://doi.org/10.1515/revic-2017-0010
P. P. Fedorov, Russ. J. Inorg. Chem. 66, 1455 (2021). https://doi.org/10.1134/S0036023621100041
P. P. Fedorov and E. V. Chernova, J. Fluorine Chem. 263, 110031 (2022). https://doi.org/10.1016/j.jfluchem.2022.110031
P. P. Fedorov, Inorg. Mater. 33, 1197 (1997).
P. P. Fedorov and L. A. Ol’khovaya, Zh. Neorg. Khim. 26, 218 (1981).
R. D. Shannon, Acta Crystallogr., Sect. A 32, 751 (1976).
S. S. Batsanov, Zh. Neorg. Khim. 18, 205 (1973).
R. E. Thoma, Advances in Molten Salt Chemistry, Ed. by J. Braunstein, vol. 3 (Plenum Press, New York, 1975).
B. G. Korshunov, V. V. Safonov, and D. V. Drobot, Fusibility Diagrams of Halide Systems of Transition Elements (Metallurgiya, Moscow, 1977) [in Russian].
B. G. Korshunov, V. V. Safonov, and D. V. Drobot, Phase Equilibria in Halide Systems (Metallurgiya, Moscow, 1979) [in Russian].
Fusibility Diagrams of Salt Systems. Directory, Ed. by V. I. Posypaiko, vols. 1, 2 (Metallurgiya, Moscow, 1977) [in Russian].
D. Babel and A. Tressaud, Inorganic Solid Fluorides. Chemistry and Physics, Ed. by P. Hagenmuller (Academic Press, Orlando, 1985).
W. Massa and D. Babel, Chem. Rev. 88, 275 (1988).
M. Leblanc, V. Maisonneuve, and A. Tressaud, Chem. Rev. 115, 1191 (2015).
Z. Mazej, J. Fluorine Chem. 265, 110073 (2023).
P. P. Fedorov, Z. I. Zhmurova, O. S. Bondareva, et al., Zh. Neorg. Khim. 39, 1010 (1994).
P. P. Fedorov, M. A. Sattarova, F. M. Spiridonov, and B. P. Sobolev, Russ. J. Inorg. Chem. 32, 90 (1987).
P. P. Fedorov, I. I. Buchinskaya, O. S. Bondareva, et al., Zh. Neorg. Khim. 40, 1380 (1995).
D. D. Ikrami, S. V. Petrov, P. P. Fedorov, et al., Zh. Neorg. Khim. 29, 1062 (1984).
D. D. Ikrami, P. P. Fedorov, A. A. Luginina, and L. A. Ol’khovaya, Zh. Neorg. Khim. 30, 1261 (1985).
P. P. Fedorov, M. N. Mayakova and V. A. Maslov, Nanosyst.: Phys. Chem. Math. 8, 830 (2017). https://doi.org/10.17586/2220-8054-2017-8-6-830-834
I. I. Buchinskaya and P. P. Fedorov, Russ. J. Inorg. Chem. 43, 1106 (1998).
L. A. Ol’khovaya, G. A. Karpenko, D. D. Ikrami, and P. P. Fedorov, Russ. J. Inorg. Chem. 36, 1639 (1991).
B. P. Sobolev, K. B. Seiranian, L. S. Garashina, and P. P. Fedorov, J. Solid State Chem. 28, 51 (1979). https://doi.org/10.1016/0022-4596(79)90057-4
P. P. Fedorov, A. A. Alexandrov, V. V. Voronov, et al., J. Am. Ceram. Soc. 104, 2836 (2021). https://doi.org/10.1111/jace.17666
P. P. Fedorov and I. I. Buchinskaya, Russ. Chem. Rev. 81, 1 (2012). https://doi.org/10.1070/RC2012v081n01ABEH004207
M. A. Kuvakin and Z. M. Novikova, Zh. Neorg. Khim. 18, 1356 (1973).
R. Roy, J. Am. Ceram. Soc. 37, 581 (1954).
A. H. M. Schrama, Physica A 68, 279 (1973).
W. Kerbe, M. Weil, F. Kubel, and H. Hagemann, Mater. Res. Bull. 39, 343 (2004).
K. Recker, F. Wallrafen, and S. Haussühl, J. Cryst. Growth 26, 97 (1974).
J. D. Donaldson and B. J. Senior, J. Chem. Soc. A11, 1821 (1967).
D. D. Ikrami, N. I. Kuznetsova, and O. I. Balashova, Proceedings of the All-Union Symposium on Chemistry of Inorganic Fluorides, Cherepovets, 1990, p. 149.
J. Portier, A. Tressaud, F. Menil, et al., J. Solid State Chem. 1, 100 (1969).
L. N. Komissarova and B. I. Pokrovskii, Dokl. Akad. Nauk SSSR 149, 599 (1963).
J.-C. Cretenet, Rev. Chim. Miner. 10, 399 (1973).
T. V. Ostrovskaya and S. A. Aminova, Zh. Neorg. Khim. 15, 657 (1970).
B. G. Müller, Z. Anorg. Allg. Chem. 555, 57 (1987).
A. A. Kostyukov and A. B. Karpov, Tr. Leningrad. Politekhn. Inst. 188, 588 (1957).
V. D. Vvedenskii, N. B. Strashko, G. A. Teterin, and E. G. Semin, Proceedings of the All-Union Symposium on Chemistry of Inorganic Fluorides, Dushanbe, 1984, p. 81.
M. Weil and F. Werner, Monatsh. Chem. 769 (2001).
J. Bandemehr, D. Baumann, M. Seibald, et al., Eur. J. Inorg. Chem. 3861 (2021). https://doi.org/10.1002/ejic.202100576
D. Reinen and F. Steffens, Z. Anorg. Allg. Chem. 441, 63 (1978).
J. Chassaing, D. Bizot, and C. Montail, Rev. Chim. Miner. 20, 753 (1983).
Z. Mazej, J. Fluorine Chem. 125, 1723 (2004).
A. I. Popov, M. D. Val’kovskii, and V. F. Sukhoverkhov, Zh. Neorg. Khim. 35, 2831 (1990).
M. Samouel, P. Salle, J. Dixuier, and P. Plurieu, C. R. Acad. Sci. C274, 955 (1972).
D. Gantar, I. Leban, B. Frlec, and J. H. Holloway, J. Chem. Soc., Dalton Trans. 2379 (1987).
C. Montail and J. Chassaing, Rev. Chim. Miner. 16, 104 (1979).
E. G. Rakov, G. G. Fedorov, and B. N. Sudarikov, Proceedings of the All-Union Symposium on Chemistry of Inorganic Fluorides, Odessa, 1972, p. 98.
Z. A. Mateiko and G. A. Bukhalova, Zh. Neorg. Khim. 7, 165 (1962).
V. T. Berezhnaya and G. A. Bukhalova, Zh. Neorg. Khim. 12, 2179 (1967).
V. T. Berezhnaya and G. A. Bukhalova, Zh. Neorg. Khim. 5, 2061 (1960).
B. V. Beznosikov, Kristallografiya 23, 113 (1978).
R. H. Nafziger, J. Am. Ceram. Soc. 54, 467 (1971).
D. N. Karimov, I. I. Buchinskaya, and N. I. Sorokin, Inorg. Mater. 55, 495 (2019). https://doi.org/10.1134/S002016851905008X
B. Müller and R. Hoppe, Mater. Res. Bull. 7, 1297 (1972).
D. Dumora, J. Ravez, and P. Hagenmuller, Bull. Soc. Chim. Fr. 4, 1301 (1970).
D. Dumora, J. Ravez, and P. Hagenmuller, Bull. Soc. Chim. Fr. 6, 2010 (1971).
G. Denes, J. Pannetier, and J. Lucas, C. R. Acad. Sci. C280, 831 (1975).
J. Ravez, R. De Pape, and P. Hagenmuller, Bull. Soc. Chim. Fr. 11, 4375 (1967).
P. P. Fedorov, Russ. J. Inorg. Chem. 57, 959 (2012). https://doi.org/10.1134/S003602361207011X
B. P. Sobolev and K. B. Seiranian, J. Solid State Chem. 39, 17 (1981).
B. P. Sobolev, The Rare Earth Trifluorides (Institut d’Estudis Catalans, Barcelona, 2000).
J. Ravez and P. Hagenmuller, Bull. Soc. Chim. Fr. 10, 3452 (1971).
J. Grannec and J. Ravez, C. R. Acad. Sci. C270, 2059 (1970).
J. Ravez, J. Grannec, and J. Portier, Rev. Chim. Miner. 8, 131 (1971).
J. Ravez and P. Hagenmuller, Bull. Soc. Chim. Fr. 7, 2545 (1967).
R. Von der Mühll and J. Ravez, Rev. Chim. Miner. 11, 652 (1974).
J. Ravez, J. Grannec, and R. Muhll, C. R. Acad. Sci. C272, 1042 (1971).
J. Ravez, M. Vassiliadis, and P. Hagenmuller, C. R. Acad. Sci. C262, 1876 (1969).
J.-C. Cretenet, C. R. Acad. Sci. C268, 945 (1969).
J. Ravez, J. Viollet, R. De Pape, and P. Hagenmuller, Bull. Soc. Chim. Fr. 4, 1325 (1967).
O. N. Breusov, G. Trapp, A. V. Novoselova, and Yu. P. Simanov, Zh. Neorg. Khim. 4, 671 (1959).
C. Fouassier, B. Latourrette, J. Portier, and P. Hagenmuller, Mater. Res. Bull. 11, 933 (1976).
R. De Pape and J. Ravez, C. R. Acad. Sci. 254, 4171 (1962).
P. Gravereau, C. Mirambet, L. Fournes, et al., Acta Crystallogr., Sect. C 46, 2294 (1990).
T. Fleischer and R. Hoppe, Z. Anorg. Allg. Chem. 490, 121 (1982).
R. Hoppe, Angew. Chem., Int. Ed. Engl. 20, 63 (1981).
E. Largeau and M. El-Ghozi, J. Fluorine Chem. 89, 223 (1998).
J. Ravez, M. Vassiliadis, R. Muhll, and P. Hagenmuller, Rev. Chim. Miner. 7, 967 (1970).
K. Feldner and R. Hoppe, Rev. Chim. Miner. 20, 351 (1983).
W. H. Zachariasen, Acta Crystallogr. 2, 388 (1949).
C. Keller and M. Salzer, J. Inorg. Nucl. Chem. 29, 2925 (1967).
I. D. Ratnikova, Yu. M. Korenev, and A. V. Novoselova, Zh. Neorg. Khim. 25, 816 (1980).
J. P. Laval, J. Solid State Chem. 309, 122962 (2022).
J. Chassaing, C. Montail, and D. Bizot, J. Solid State Chem. 43, 327 (1982).
B. Frlec, D. Gantar, and J. H. Holloway, J. Fluorine Chem. 19, 485 (1982).
T. Bunic, M. Tramsek, E. Goreshnik, et al., Solid State Sci. 9, 88 (2007).
P. P. Fedorov, Kristallografiya 42, 1141 (1997).
P. P. Fedorov, Neorg. Mater. 33, 1415 (1997).
ACKNOWLEDGMENTS
The facilities of the Shared Facilities Center of the Prokhorov General Physics Institute, Russian Academy of Sciences, and the “Materials Science” Shared Facilities Center of the Ogarev Mordovian State University were used in the work.
Funding
This work was fulfilled as part of the strategic project “New Generation Materials and Energy Saving” implemented at the Ogarev Mordovian State University in accordance with the “Priority 2030” program and in accordance with the scientific work plan of the Prokhorov General Physics Institute, Russian Academy of Sciences (theme “Kvant”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fedorov, P.P., Pynenkov, A.A., Uslamina, M.A. et al. Phase Diagram of the MgF2–SrF2 System and Interactions of Magnesium and Strontium Fluorides with Other Fluorides. Russ. J. Inorg. Chem. 68, 1789–1798 (2023). https://doi.org/10.1134/S0036023623602325
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023623602325