Abstract
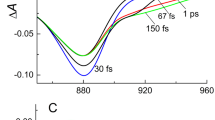
In green photosynthetic bacteria, light is absorbed by bacteriochlorophyll (BChl) c/d/e oligomers, which are located in chlorosomes – unique structures created by Nature to collect the energy of very weak light fluxes. Using coherent femtosecond spectroscopy at cryogenic temperature, we detected and studied low-frequency vibrational motions of BChl c oligomers in chlorosomes of the green bacteria Chloroflexus (Cfx.) aurantiacus. The objects of the study were chlorosomes isolated from the bacterial cultures grown under different light intensity. It was found that the Fourier spectrum of low-frequency coherent oscillations in the Qy band of BChl c oligomers depends on the light intensity used for the growth of bacteria. It turned out that the number of low-frequency vibrational modes of chlorosomes increases as illumination under which they were cultivated decreases. Also, the frequency range within which these modes are observed expands, and frequencies of the most modes change. Theoretical modeling of the obtained data and analysis of the literature led to conclusion that the structural basis of Cfx. aurantiacus chlorosomes are short linear chains of BChl c combined into more complex structures. Increase in the length of these chains in chlorosomes grown under weaker light leads to the observed changes in the spectrum of vibrations of BChl c oligomers. This increase is an effective mechanism for bacteria adaptation to changing external conditions.






Similar content being viewed by others
Abbreviations
- ΔA:
-
absorbance changes (light-dark)
- BChl:
-
bacteriochlorophyll
- Cb. :
-
Chlorobium
- Cfx. :
-
Chloroflexus
- Chl:
-
chlorophyll
- Rba. :
-
Rhodobacter
References
Clayton, R. (1980) Photosynthesis: physical mechanisms and chemical patterns, Cambridge University Press, USA.
Mirkovic. T., Ostroumov, E., Anna, J., van Grondelle, R., Govindjee, and Scholes, G. (2017) Light absorption and energy transfer in the antenna complexes of photosynthetic organisms, Chem. Rev., 117, 249-293, https://doi.org/10.1021/acs.chemrev.6b00002.
Frigaard, N.-U., and Bryant, D. (2006) Chlorosomes: antenna organelles in green photosynthetic bacteria, in Complex intracellular structures in prokaryotes. Microbiology monographs (Shively, J. M., ed) vol. 2, Springer, Berlin, pp. 79-114, https://doi.org/10.1007/7171_021.
Krasnovsky, A., and Bystrova, M. (1980) Self-assembly of chlorophyll aggregated structures, BioSystems, 12, 181-194, https://doi.org/10.1016/0303-2647(80)90016-7.
Smith, K., Kehres, L., and Fajer, J. (1983) Aggregation of bacteriochlorophylls c, d or e. Models for the antenna chlorophylls of green and brown photosynthetic bacteria, J. Am. Chem. Soc., 105, 1387-1389, https://doi.org/10.1021/ja00343a062.
Van Dorssen, R. J., Vasmel, H., and Amesz, J. (1986) Pigment organization and energy transfer in the green photosynthetic bacterium Chloroflexus aurantiacus. II. The chlorosome, Photosynth. Res., 9, 33-45, https://doi.org/10.1007/BF00029729.
Fetisova, Z., Freiberg, A., and Timpmann, K. (1988) Long-range molecular order as an efficient strategy for light harvesting in photosynthesis, Nature (London), 334, 633-634, https://doi.org/10.1038/334633a0.
Fetisova, Z., and Mauring, K. (1992) Experimental evidence of oligomeric organization of antenna bacteriochlorophyll c in green bacterium Chloroflexus aurantiacus by spectral hole burning, FEBS Lett., 307, 371-374, https://doi.org/10.1016/0014-5793(92)80715-S.
Fetisova, Z., and Mauring, K. (1993) Spectral hole burning study of intact cells of green bacterium Chlorobium limicola, FEBS Lett., 323, 159-162, https://doi.org/10.1016/0014-5793(93)81470-K.
Fetisova, Z., Mauring, K., and Taisova, A. (1994) Strongly exciton coupled BChl e chromophore system in chlorosomal antenna of intact cells of green bacterium Chlorobium phaeovibrioides: A spectral hole burning study, Photosynth. Res., 41, 205-210, https://doi.org/10.1007/BF02184161.
Fetisova, Z., Freiberg, A., Mauring, K., Novoderezhkin, V., Taisova, A., and Timpmann, K. (1996) Excitation energy transfer in chlorosomes of green bacteria: theoretical and experimental studies, Biophys. J., 71, 995-1010, https://doi.org/10.1016/S0006-3495(96)79301-3.
Prokhorenko, V. I., Steensgaard, D. B., and Holzwarth, A. R. (2000) Exciton dynamics in the chlorosomal antennae of the green bacteria Chloroflexus aurantiacus and Chlorobium tepidum, Biophys. J., 79, 2105-2120, https://doi.org/10.1016/S0006-3495(00)76458-7.
Mauring, K., Novoderezhkin, V., Taisova, A., and Fetisova, Z. (1999) Exciton levels structure of antenna bacteriochlorophyll c aggregates in the green bacterium Chloroflexus aurantiacus as probed by 1.8-293 K fluorescence spectroscopy, FEBS Lett., 456, 239-242, https://doi.org/10.1016/S0014-5793(99)00953-9.
Martiskainen, J., Linnanto, J., Kananavičius, R., Lehtovuori, V., and Korppi-Tommola, J. (2009) Excitation energy transfer in isolated chlorosomes from Chloroflexus aurantiacus, Chem. Phys. Lett., 477, 216-220, https://doi.org/10.1016/j.cplett.2009.06.080.
Martiskainen, J., Linnanto, J., Aumanen, V., Myllyperkiö, P., and Korppi-Tommola, J. (2012) Excitation energy transfer in isolated chlorosomes from Chlorobaculum tepidum and Prosthecochloris aestuarii, Photochem. Photobiol., 88, 675-683, https://doi.org/10.1111/j.1751-1097.2012.01098.x.
Linnanto, J. V., and Korppi-Tommola, J. E. I. (2012) Exciton description of excitation energy transfer in the photosynthetic units of green sulfur bacteria and filamentous anoxygenic phototrophs, J. Phys. Chem. B, 117, 11144-11161, https://doi.org/10.1021/jp4011394.
Staehelin, L., Golecki, J., Fuller, R., and Drews, G. (1978) Visualization of the supramolecular architecture of chlorosomes (Chlorobium type vesicles) in freeze-fractured cells of Chloroflexus aurantiacus, Arch. Microbiol., 119, 269-277, https://doi.org/10.1007/BF00405406.
Sprague, S., Staehelin, L., DiBartolomeis, M., and Fuller, R. (1981) Isolation and development of chlorosomes in the green bacterium Chloroflexus aurantiacus, J. Bacteriol., 147, 1021-1031, https://doi.org/10.1128/jb.147.3.1021-1031.1981.
Psencik, J., Ikonen, T. P., Laurinmaki, P., Merckel, M. C., Butcher, S. J., Serimaa, R. E., and Tuma, R. (2004) Lamellar organization of pigments in chlorosomes, the light harvesting complexes of green photosynthetic bacteria, Biophys. J., 87, 1165-1172, https://doi.org/10.1529/biophysj.104.040956.
Günther, L., Jendrny, M., Bloemsma, E., Tank, M., Oostergetel, G., Bryant, D., Knoester, J., and Köhler, J. (2016) Structure of light-harvesting aggregates in individual chlorosomes, J. Phys. Chem. B, 120, 5367-5376, https://doi.org/10.1021/acs.jpcb.6b03718.
Sawaya, N., Huh, J., Fujita, T., Saikin, S., and Aspuru-Guzik, A. (2015) Fast delocalization leads to robust long-range excitonic transfer in a large quantum chlorosome model, Nano Lett., 15, 1722-1729, https://doi.org/10.1021/nl504399d.
Fujita, T., Huh, J., Saikin, S., Brookes, J., and Aspuru-Guzik, A. (2014) Theoretical characterization of excitation energy transfer in chlorosome light-harvesting antennae from green sulfur bacteria, Photosynth. Res., 120, 273-289, https://doi.org/10.1007/s11120-014-9978-7.
Yakovlev, A. G., Taisova, A. S., and Fetisova, Z. G. (2021) Utilization of blue-green light by chlorosomes from the photosynthetic bacterium Chloroflexus aurantiacus: Ultrafast excitation energy conversion and transfer, Biochim. Biophys. Acta Bioenergetics, 1862, 148396, https://doi.org/10.1016/j.bbabio.2021.148396.
Savikhin, S., Zhu, Y., Blankenship, R. E., and Struve, W. S. (1996) Intraband energy transfers in the BChl c antenna of chlorosomes from the green photosynthetic bacterium Chloroflexus aurantiacus, J. Phys. Chem., 100, 17978-17980, https://doi.org/10.1021/jp961752b.
Savikhin, S., Zhu, Y., Lin, S., Blankenship, R. E., and Struve, W. S. (1994) Femtosecond spectroscopy of chlorosome antennas from the green photosynthetic bacterium Chloroflexus aurantiacus, J. Phys. Chem., 98, 10322-10334, https://doi.org/10.1021/j100091a056.
Cherepy, N. J., Du Mei, Holzwarth, A. R., and Mathies, R. A. (1996) Near-infrared resonance Raman spectra of chlorosomes: probing nuclear coupling in electronic energy transfer, J. Phys. Chem., 100, 4662-4671, https://doi.org/10.1021/jp952992e.
Klevanik, A. V. (2001) Low frequency vibrations of bacteriochlorophyll, Optics Spectroscopy, 90, 55-66, https://doi.org/10.1134/1.1343547.
Yakovlev, A. G., Taisova, A. S., Shuvalov, V. A., and Fetisova, Z. G. (2018) Estimation of the bacteriochlorophyll c oligomerisation extent in Chloroflexus aurantiacus chlorosomes by very low-frequency vibrations of the pigment molecules: A new approach, Biophys. Chem., 240, 1-8, https://doi.org/10.1016/j.bpc.2018.05.004.
Pierson, B., and Castenholz, R. (1974) Studies of pigments and growth in Chloroflexus aurantiacus, a phototrophic filamentous bacterium, Arch. Microbiol., 100, 283-305, https://doi.org/10.1007/BF00446324.
Taisova, A. S., Keppen, O. I., Lukashev, E. P., Arutyunyan, A. M., and Fetisova, Z. G. (2002) Study of the chlorosomal antenna of the green mesophilic filamentous bacterium Oscillochloris trichoides, Photosynth. Res., 74, 73-85, https://doi.org/10.1023/A:1020805525800.
Trubetzkov, D. I., and Rojnev, A. G. (2001) Linear Vibrations and Waves [in Russian], Fizmatlit, Moscow.
Yakovlev, A. G., Taisova, A. S., and Fetisova, Z. G. (2020) Q-band hyperchromism and B-band hypochromism of bacteriochlorophyll c as a tool for investigation of the oligomeric structure of chlorosomes of the green photosynthetic bacterium Chloroflexus aurantiacus, Photosynth. Res., 146, 95-108, https://doi.org/10.1007/s11120-019-00707-9.
Yakovlev, A., Taisova, A., Arutyunyan, A., Shuvalov, V., and Fetisova, Z. (2017) Variability of aggregation extent of light-harvesting pigments in peripheral antenna of Chloroflexus aurantiacus, Photosynth. Res., 133, 343-356, https://doi.org/10.1007/s11120-017-0374-y.
Glinka, N. L. (1975) General Chemistry [in Russian], Chemistry, Leningrad.
Savikhin, S., van Noort, P. I., Zhu, Y., Lin, S., Blankenship, R. E., and Struve, W. S. (1995) Ultrafast energy transfer in light-harvesting chlorosomes from the green sulfur bacterium Chlorobium tepidum, Chem. Phys., 194, 245-258, https://doi.org/10.1016/0301-0104(95)00019-K.
Yakovlev, A. G., Shkuropatov, A. Ya., and Shuvalov, V. A. (2002) Nuclear wavepacket motion between P* and P+BA– potential surfaces with a subsequent electron transfer to HA in bacterial reaction centers at 90 K. Electron transfer pathway, Biochemistry, 41, 14019-14027, https://doi.org/10.1021/bi020250n.
Meneghin, E., Leonardo, C., Volpato, A., Bolzonello, L., and Collini, E. (2017) Mechanistic insight into internal conversion process within Q-bands of chlorophyll a, Sci. Rep., 7, 11389, https://doi.org/10.1038/s41598-017-11621-2.
Lutz, M. (1977) Antenna chlorophyll in photosynthetic membranes. A study by resonance Raman spectroscopy, Biochim. Biophys. Acta, 460, 408-430, https://doi.org/10.1016/0005-2728(77)90081-0.
Novoderezhkin, V. I., Taisova, A. S., and Fetisova, Z. G. (2001) Unit building block of the oligomeric chlorosomal antenna of the green photosynthetic bacterium Chloroflexus aurantiacus: modeling of nonlinear optical spectra, Chem. Phys. Lett., 335, 234-240, https://doi.org/10.1016/S0009-2614(01)00045-8.
Acknowledgments
The authors express their deep gratitude to the late Professor V. A. Shuvalov for general support and constant attention to the work.
Funding
The study was financially supported by the State Budget Project no. AAAA-A17-117120540070-0 (“Photobiophysics of Solar Energy Conversion in Living Systems”).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to the work.
Corresponding author
Ethics declarations
The authors declare no conflicts of interests in financial or any other sphere. This article does not contain description of research involving human participants or animals performed by any of the authors.
Additional information
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yakovlev, A.G., Taisova, A.S. & Fetisova, Z.G. Low-Frequency Oscillations of Bacteriochlorophyll Oligomers in Chlorosomes of Photosynthetic Green Bacteria. Biochemistry Moscow 88, 2084–2093 (2023). https://doi.org/10.1134/S0006297923120118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923120118