Abstract
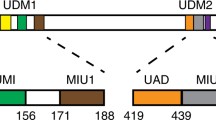
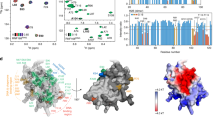
Genome stability is critical for normal functioning of cells, it depends on accuracy of DNA replication, chromosome segregation, and DNA repair. Cellular defense mechanisms against DNA damage are important for preventing cancer development and aging. The E3 ubiquitin ligase RNF168 of the RING superfamily is an essential component of the complex responsible for ubiquitination of the H2A/H2A.X histones near DNA double-strand breaks, which is a key step in attracting repair factors to the damage site. In this study, we unequivocally showed that RNF168 does not have the ability to directly distinguish architecture of polyubiquitin chains, except for the tropism of its two ubiquitin-binding domains UDM1/2 to K63 ubiquitin chains. Analysis of intracellular chromatosomal environment of the full-length RNF168 and its domains using the ligand-induced bioluminescence resonance energy transfer (BRET) revealed that the C-terminal part of UDM1 is associated with the K63 ubiquitin chains; RING and the N-terminal part of UDM2 are sterically close to the K63- and K48-ubiquitin chains, while the C-terminal part of UDM1 is co-localized with all possible ubiquitin variants. Our observations together with the available structural data suggest that the C-terminal part of UDM1 binds the K63 polyubiquitin chains on the linker histone H1; RING and the N-terminal part of UDM2 are located in the central part of nucleosome and sterically close to H1 and K48-ubiquitinated alternative substrates of RNF168, such as JMJD2A/B demethylases, while the C-terminal part of UDM1 is in the region of activated ubiquitin residue associated with E2 ubiquitin ligase, engaged by RNF168.




Similar content being viewed by others
Abbreviations
- DSB:
-
double-strand DNA breaks
- HR:
-
homologous recombination
- LR:
-
leucine-arginine
- NanoBRET:
-
bioluminescence resonance energy transfer-based assay that uses NanoLuc® Luciferase
- SPR:
-
surface plasmon resonance
References
Khanna, K. K., and Jackson, S. P. (2001) DNA double-strand breaks: signaling, repair and the cancer connection, Nat. Genet., 27, 247-254, https://doi.org/10.1038/85798.
Jackson, S. P., and Bartek, J. (2009) The DNA-damage response in human biology and disease, Nature, 461, 1071-1078, https://doi.org/10.1038/nature08467.
Ceccaldi, R., Rondinelli, B., and D’Andrea, A. D. (2016) Repair pathway choices and consequences at the double-strand break, Trends Cell Biol., 26, 52-64, https://doi.org/10.1016/j.tcb.2015.07.009.
Price, B. D., and D’Andrea, A. D. (2013) Chromatin remodeling at DNA double-strand breaks, Cell, 152, 1344-1354, https://doi.org/10.1016/j.cell.2013.02.011.
Kim, J. J., Lee, S. Y., and Miller, K. M. (2019) Preserving genome integrity and function: the DNA damage response and histone modifications, Crit. Rev. Biochem. Mol. Biol., 54, 208-241, https://doi.org/10.1080/10409238.2019.1620676.
Bacheva, A. V., Gotmanova, N. N., Belogurov, A. A., and Kudriaeva, A. A. (2021) Control of genome through variative nature of histone-modifying ubiquitin ligases, Biochemistry (Moscow), 86, S71-S95, https://doi.org/10.1134/S0006297921140066.
Ciechanover, A. (2015) The unravelling of the ubiquitin system, Nat. Rev. Mol. Cell. Biol., 16, 322-324, https://doi.org/10.1038/nrm3982.
Kudriaeva, A. A., and Belogurov, A. A. (2019) Proteasome: a nanomachinery of creative destruction, Biochemistry (Moscow), 84, 159-192, https://doi.org/10.1134/S0006297919140104.
Kudriaeva, A. A., Livneh, I., Baranov, M. S., Ziganshin, R. H., Tupikin, A. E., Zaitseva, S. O., Kabilov, M. R., Ciechanover, A., and Belogurov, A. A. Jr. (2021) In-depth characterization of ubiquitin turnover in mammalian cells by fluorescence tracking, Cell. Chem. Biol., 28, 1192-1205, https://doi.org/10.1016/j.chembiol.2021.02.009.
Chen, Z. J., and Sun, L. J. (2009) Nonproteolytic functions of ubiquitin in cell signaling, Mol. Cell, 33, 275-286, https://doi.org/10.1016/j.molcel.2009.01.014.
Jackson, S. P., and Durocher, D. (2013) Regulation of DNA damage responses by ubiquitin and SUMO, Mol. Cell, 49, 795-807, https://doi.org/10.1016/j.molcel.2013.01.017.
Iwai, K., and Tokunaga, F. (2009) Linear polyubiquitination: a new regulator of NF-κB activation, EMBO Rep., 10, 706-713, https://doi.org/10.1038/embor.2009.144.
Matsumoto, M. L., Wickliffe, K. E., Dong, K. C., Yu, C., Bosanac, I., Bustos, D., Phu, L., Kirkpatrick, D. S., Hymowitz, S. G., Rape, M., Kelley, R. F., and Dixit, V. M. (2010) K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody, Mol. Cell, 39, 477-484, https://doi.org/10.1016/j.molcel.2010.07.001.
Uckelmann, M., and Sixma, T. K. (2017) Histone ubiquitination in the DNA damage response, DNA Repair (Amst), 56, 92-101, https://doi.org/10.1016/j.dnarep.2017.06.011.
Nishi, R. (2017) Balancing act: To be, or not to be ubiquitylated, Mutat. Res., 803-805, 43-50, https://doi.org/10.1016/j.mrfmmm.2017.07.006.
Gudjonsson, T., Altmeyer, M., Savic, V., Toledo, L., Dinant, C., Grøfte, M., Bartkova, J., Poulsen, M., Oka, Y., Bekker-Jensen, S., Mailand, N., Neumann, B., Heriche, J. K., Shearer, R., Saunders, D., Bartek, J., Lukas, J., and Lukas, C. (2012) TRIP12 and UBR5 suppress spreading of chromatin ubiquitylation at damaged chromosomes, Cell, 150, 697-709, https://doi.org/10.1016/j.cell.2012.06.039.
Gatti, M., Pinato, S., Maiolica, A., Rocchio, F., Prato, M. G., Aebersold, R., and Penengo, L. (2015) RNF168 promotes noncanonical K27 ubiquitination to signal DNA damage, Cell Rep., 10, 226-238, https://doi.org/10.1016/j.celrep.2014.12.021.
Kudriaeva, A. A., Lipkin, V. M., and Belogurov, A. A. (2020) Topological features of histone H2A monoubiquitination, Dokl. Biochem. Biophys., 493, 193-197, https://doi.org/10.1134/S1607672920040079.
Kelliher, J., Ghosal, G., and Leung, J. W. C. (2022) New answers to the old RIDDLE: RNF168 and the DNA damage response pathway, FEBS J., 289, 2467-2480, https://doi.org/10.1111/febs.15857.
Pinato, S., Gatti, M., Scandiuzzi, C., Confalonieri, S., and Penengo, L. (2011) UMI, a novel RNF168 ubiquitin binding domain involved in the DNA damage signaling pathway, Mol. Cell. Biol., 31, 118-126, https://doi.org/10.1128/mcb.00818-10.
Takahashi, T. S., Hirade, Y., Toma, A., Sato, Y., Yamagata, A., Goto-Ito, S., Tomito, A., Nakada, S., and Fukai, S. (2018) Structural insights into two distinct binding modules for Lys63-linked polyubiquitin chains in RNF168, Nat. Commun., 9, 170, https://doi.org/10.1038/s41467-017-02345-y.
Kitevski-LeBlanc, J., Fradet-Turcotte, A., Kukic, P., Wilson, M. D., Portella, G., Yuwen, T., Panier, S., Duan, S., Canny, M. D., van Ingen, H., Arrowsmith, C. H., Rubinstein, J. L., Vendruscolo, M., Durocher, D., and Kay, L. E. (2017) The RNF168 paralog RNF169 defines a new class of ubiquitylated histone reader involved in the response to DNA damage, Elife, 6, e23872, https://doi.org/10.7554/eLife.23872.
Pinato, S., Scandiuzzi, C., Arnaudo, N., Citterio, E., Gaudino, G., and Penengo, L. (2009) RNF168, a new RING finger, MIU-containing protein that modifies chromatin by ubiquitination of histones H2A and H2AX, BMC Mol. Biol., 10, 55, https://doi.org/10.1186/1471-2199-10-55.
Horn, V., Uckelmann, M., Zhang, H., Eerland, J., Aarsman, I., le Paige, U. B., Davidovich, C., Sixma, T. K., and van Ingen, H. (2019) Structural basis of specific H2A K13/K15 ubiquitination by RNF168, Nat. Commun., 10, 1751, https://doi.org/10.1038/s41467-019-09756-z.
Machleidt, T., Woodroofe, C. C., Schwinn, M. K., Méndez, J., Robers, M. B., Zimmerman, K., Otto, P., Daniels, D. L., Kirkland, T. A., and Wood, K. V (2015) NanoBRET – a novel BRET platform for the analysis of protein-protein interactions, ACS Chem. Biol., 10, 1797-1804, https://doi.org/10.1021/acschembio.5b00143.
Weihs, F., Wang, J., Pfleger, K. D. G., and Dacres, H. (2020) Experimental determination of the bioluminescence resonance energy transfer (BRET) Förster distances of NanoBRET and red-shifted BRET pairs, Anal. Chim. Acta X, 6, 100059, https://doi.org/10.1016/j.acax.2020.100059.
Bednar, J., Garcia-Saez, I., Boopathi, R., Cutter, A. R., Papai, G., Reymer, A., Syed, S. H., Lone, I. N., Tonchev, O., Crucifix, C., Menoni, H., Papin, C., Skoufias, D. A., Kurumizaka, H., Lavery, R., Hamiche, A., Hayes, J. J., Schultz, P., Angelov, D., Petosa, C., and Dimitrov, S. (2017) Structure and dynamics of a 197 bp nucleosome in complex with linker histone H1, Mol. Cell, 66, 384-397.e8, https://doi.org/10.1016/j.molcel.2017.04.012.
Panier, S., Ichijima, Y., Fradet-Turcotte, A., Leung, C. C. Y., Kaustov, L., Arrowsmith, C. H., and Durocher, D. (2012) Tandem protein interaction modules organize the ubiquitin-dependent response to DNA double-strand breaks, Mol. Cell, 47, 383-395, https://doi.org/10.1016/j.molcel.2012.05.045.
Thorslund, T., Ripplinger, A., Hoffmann, S., Wild, T., Uckelmann, M., Villumsen, B., Narita, T., Sixma, T. K., Choudhary, C., Bekker-Jensen, S., and Mailand, N. (2015) Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage, Nature, 527, 389-393, https://doi.org/10.1038/nature15401.
Mattiroli, F., Uckelmann, M., Sahtoe, D. D., van Dijk, W. J., and Sixma, T. K. (2014) The nucleosome acidic patch plays a critical role in RNF168-dependent ubiquitination of histone H2A, Nat. Commun., 5, 3291, https://doi.org/10.1038/ncomms4291.
Mallette, F. A., and Richard, S. (2012) K48-linked ubiquitination and protein degradation regulate 53BP1 recruitment at DNA damage sites, Cell Res., 22, 1221-1223, https://doi.org/10.1038/cr.2012.58.
Funding
This work was financially supported by the Russian Science Foundation (grant no. 21-74-10154).
Author information
Authors and Affiliations
Contributions
A.A.K. and A.A.B. concept and supervision of the study; A.A.K., L.A.Ya., G.A.S. and V.I.V. conducting experiments; A.A.K., V.M.L. and A.A.B. discussing results of the study; A.A.K. and A.A.B. writing text of the paper; A.A.K. and A.A.B. editing text of the paper.
Corresponding author
Ethics declarations
The authors declare no conflict of interests in financial or any other sphere. This paper does not describe any studies involving human participant or animals performed by any of the authors.
Additional information
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kudriaeva, A.A., Yakubova, L.A., Saratov, G.A. et al. Topology of Ubiquitin Chains in the Chromatosomal Environment of the E3 Ubiquitin Ligase RNF168. Biochemistry Moscow 88, 2063–2072 (2023). https://doi.org/10.1134/S000629792312009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629792312009X