Abstract
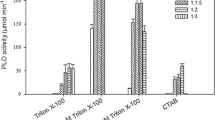
The study of many membrane enzymes in an aqueous medium is difficult due to the loss of their catalytic activity, which makes it necessary to use membrane-like systems, such as reverse micelles of surfactants in nonpolar organic solvents. However, it should be taken into account that the micelles are a simplified model of natural membranes, since membranes contain many different components, a significant part of which are phospholipids. In this work, we studied impact of the main phospholipids, phosphatidylcholine (PC) and phosphatidylethanolamine (PE), on activity of the membrane enzymes using galactonolactone oxidase from Trypanosoma cruzi (TcGAL) and L-galactono-1,4-lactone dehydrogenase from Arabidopsis thaliana (AtGALDH) as examples. Effect of the structure (and charge) of the micelle-forming surfactant itself on the activity of both enzymes has been studied using an anionic surfactant (AOT), a neutral surfactant (Brij-96), and a mixture of cationic and anionic surfactants (CTAB and AOT) as examples. The pronounced effect of addition of PC and PE lipids on the activity of AtGALDH and TcGAL has been detected, which manifests as increase in catalytic activity and significant change in the activity profile. This can be explained by formation of the tetrameric form of enzymes and/or protein–lipid complexes. By varying composition and structure of the micelle-forming surfactants (AOT, CTAB, and Brij-96) it has been possible to change catalytic properties of the enzyme due to effect of the surfactant on the micelle size, lipid mobility, charge, and rigidity of the matrix itself.






Similar content being viewed by others
Abbreviations
- AL:
-
D-arabinono-1,4-lactone
- AOT:
-
bis-2-ethylhexyl sulfosuccinate, anionic SAA
- AtGALDH:
-
L-galactono-1,4-lactone dehydrogenase from Arabidopsis thaliana
- CTAB:
-
cetyltrimethylammonium bromide, cationic SAA
- DCPIP:
-
2,6-dichlorophenolindophenol
- EA:
-
electron acceptor
- GL:
-
L-galactono-1,4-lactone
- PC:
-
phosphatidylcholine
- PE:
-
phosphatidylethanolamine
- PMS:
-
phenazine methosulfate
- SAA:
-
surface active agents
- TcGAL:
-
galactonolactone oxidase from Trypanosoma cruzi
- W0 :
-
the hydration degree
References
Kudryashova, E. V., Leferink, N. G. H., Slot, I. G. M., and van Berkel, W. J. H. (2011) Galactonolactone oxidoreductase from Trypanosoma cruzi employs a FAD cofactor for the synthesis of vitamin C, Biochim. Biophys. Acta, 1814, 545-552, https://doi.org/10.1016/j.bbapap.2011.03.001.
Quiñones, W., Acosta, H., Gonçalves, C. S., Motta, M. C. M., Gualdrón-López, M., and Michels, P. A. M. (2020) Structure, properties, and function of glycosomes in Trypanosoma cruzi, Front. Cell. Infect. Microbiol., 10, 25, https://doi.org/10.3389/fcimb.2020.00025.
Wilkinson, S. R., Prathalingam, S. R., Taylor, M. C., Horn, D., and Kelly, J. M. (2005) Vitamin C biosynthesis in trypanosomes: a role for the glycosome, Proc. Natl. Acad. Sci. USA, 102, 11645-11650, https://doi.org/10.1073/pnas.0504251102.
Logan, F. J., Taylor, M. C., Wilkinson, S. R., Kaur, H., and Kelly, J. M. (2007) The terminal step in vitamin C biosynthesis in Trypanosoma cruzi is mediated by a FMN-dependent galactonolactone oxidase, Biochem. J., 407, 419-426, https://doi.org/10.1042/BJ20070766.
Chudin, A. A., and Kudryashova, E. V. (2022) Improved enzymatic assay and inhibition analysis of redox membranotropic enzymes, AtGALDH and TcGAL, using a reversed micellar system, Analytica, 3, 36-53, https://doi.org/10.3390/analytica3010004.
Leferink, N. G. H., Heuts, D. P. H. M., Fraaije, M. W., and van Berkel, W. J. H. (2008) The growing VAO flavoprotein family, Arch. Biochem. Biophys., 474, 292-301, https://doi.org/10.1016/j.abb.2008.01.027.
Khmelnitsky, Y. L., Levashov, A. V., Klyachko, N. L., and Martinek, K. (1984) A microheterogeneous medium for chemical (enzymic) reactions based on a colloidal solution of water in an organic solvent, Russ. Chem. Rev., 53, 319-331, https://doi.org/10.1070/RC1984v053n04ABEH003052.
Klyachko, N. L., Shchedrina, V. A., Efimov, A. V., Kazakov, S. V., Gazaryan, I. G., Kristal, B. S., and Brown, A. M. (2005) pH-dependent substrate preference of pig heart lipoamide dehydrogenase varies with oligomeric state: response to mitochondrial matrix acidification, J. Biol. Chem., 280, 16106-16114, https://doi.org/10.1074/jbc.M414285200.
Kudryashova, E. V., Bronza, V. L., Vinogradov, A. A., Kamyshny, A., Magdassi, S., and Levashov, A. V. (2011) Regulation of acid phosphatase in reverse micellar system by lipids additives: structural aspects, J. Colloid Interface Sci., 353, 490-497, https://doi.org/10.1016/j.jcis.2010.09.072.
De Azevedo-Martins, A. C., Ocaña, K., de Souza, W., de Vasconcelos, A. T. R., Teixeira, M. M. G., Camargo, E. P., Alves, J. M. P., and Motta, M. C. M. (2022) The importance of glycerophospholipid production to the mutualist symbiosis of trypanosomatids, Pathogens, 11, 41, https://doi.org/10.3390/pathogens11010041.
Oliveira, M. M., Timm, S. L., and Costa, S. C. G. (1977) Lipid composition of Trypanosoma cruzi, Comp. Biochem. Physiol. B Comp. Biochem., 58, 195-199, https://doi.org/10.1016/0305-0491(77)90109-2.
Urbina, J. A., Marchan, E., Lazardi, K., Visbal, G., Apitz-Castro, R., Gil, F., Aguirre, T., Piras, M. M., and Piras, R. (1993) Inhibition of phosphatidylcholine biosynthesis and cell proliferation in Trypanosoma cruz by ajoene, an antiplatelet compound isolated from garlic, Biochem. Pharmacol., 45, 2381-2387, https://doi.org/10.1016/0006-2952(93)90217-k.
Shome, A., Roy, S., and Kumar, P. (2007) Nonionic surfactants: a key to enhance the enzyme activity at cationic reverse micellar interface, Langmuir, 23, 4130-4136, https://doi.org/10.1021/la062804j.
Leferink, N. G. H., van Den Berg, W. A. M., and van Berkel, W. J. H. (2008) L-Galactono-γ-lactone dehydrogenase from Arabidopsis thaliana, a flavoprotein involved in vitamin C biosynthesis, FEBS J., 275, 713-726, https://doi.org/10.1111/j.1742-4658.2007.06233.x.
Khmelnitsky, Y. L., Levashov, A. V., Klyachko, N. L., Chernyak, V. Y., Martinek, K. (1982) Enzymes incorporated into reversed micelles of surfactants in organic solvents. Study of the protein-aerosol OT-H2O-octane system by sedimentation analysis [in Russian], Biokhimiya, 47, 86-99.
Levashov, A. V., Khmelnitsky, Y. L., Klyachko, N. L., Chernyak, V. Y., and Martinek, K. (1981) Ultracantrifugation of reversed micelles in organic solvent: new approach to determination of molecular weight and effective size of proteins, Anal. Biochem., 118, 42-46, https://doi.org/10.1016/0003-2697(81)90153-6.
Krasnopevtseva, M. K., Belik, V. P., Bogdanov, A. A., Semenova, I. V., Smolin, A. G., and Vasyutinskii, O. S. (2020) Decay times and anisotropy in polarized fluorescence of flavin adenine dinucleotide determined with subnanosecond resolution, Tech. Phys. Lett., 46, 614-616, https://doi.org/10.1134/S1063785020060218.
Kudryashova, E. V., Visser, A. J. W. G., and van Berkel, W. J. H. (2008) Monomer formation and function of p-Hydroxybenzoate hydroxylase in reverse micelles and dimethylsulfoxide/water mixtures, ChemBioChem, 9, 413-419, https://doi.org/10.1002/cbic.200700267.
Levashov, A. V., and Klyachko, N. L. (2001) Reverse micellar systems, Methods Biotechnol., 15, 575-586, https://doi.org/10.1385/1-59259-112-4:575.
Tsui, F. C., Ojcius, D. M., and Hubbell, W. L. (1986) The intrinsic pKa values for phosphatidylserine and phosphatidylethanolamine in phosphatidylcholine host bilayers, Biophys. J., 49, 459-468, https://doi.org/10.1016/S0006-3495(86)83655-4.
Levashov, A. V., Ugolnikova, A. V., Ivanov, M. V., and Klyachko, N. L. (1997) Formation of homo- and heterooligomeric supramolecular structures by D-glyceraldehyde-3-phosphate dehydrogenase and lactate dehydrogenase in reversed micelles of aerosol OT in octane, Biochem. Mol. Biol. Int., 42, 527-534, https://doi.org/10.1080/15216549700202931.
Chatterley, A. S., Laity, P., Holland, C., Weidner, T., Woutersen, S., and Giubertoni, G. (2022) Broadband multidimensional spectroscopy identifies the amide II vibrations in silkworm films, Molecules, 27, 6275, https://doi.org/10.3390/molecules27196275.
Kudryashova, E. V., Gladilin, A. K., and Levashov, A. V. (2002) Proteins in supramolecular ensembles: investigation of structure with time-resolved fluorescence anisotropy, Uspekhi Biol. Khimii, 42, 257-294.
Jonas, A., and Drengler, S. M. (1977) Fluorescence polarization studies of human and rhesus a-i apolipoproteins and their complexes with phosphatidylcholine, Biochem. Biophys. Res. Commun., 78, 1424-1430, https://doi.org/10.1016/0006-291x(77)91451-6.
Lolkema, J. S., and Slotboom, D. J. (2015) The Hill analysis and co-ion-driven transporter kinetics, J. Gen. Physiol., 145, 565-574, https://doi.org/10.1085/jgp.201411332.
Azimi, M., Nafissi-Varcheh, N., Faramarzi, M. A., and Aboofazeli, R. (2016) Laccase activity in CTAB-based water-in-oil microemulsions, Iran J. Pharm. Res., 15, 441-452.
Kabanov, A. V., Nametkin, S. N., and Levashov, A. V. (1990) The principal difference in regulation of the catalytic activity of water-soluble and membrane forms of enzymes in reversed micelles. γ-Glutamyltransferase and aminopeptidase, FEBS Lett., 267, 236-238, https://doi.org/10.1016/0014-5793(90)80933-a.
Yang, Z., and Robb, D. A. (2005) Tyrosinase activity in reversed micelles, Biocatal. Biotransform., 23, 423-430, https://doi.org/10.1080/10242420500387433.
Gupte, A., Nagarajan, R., and Kilara, A. (1995) Enzymatic oxidation of cholesterol in reverse micelles, Ind. Eng. Chem. Res., 34, 2910-2922, https://doi.org/10.1021/ie00047a045.
Chudin, A. A., Zlotnikov, I. D., Krylov, S. S., Semenov, V. V., and Kuryashova, E. V. (2023) Allylpolyalkoxybenzene inhibitors of galactonolactone oxidase from Trypanosoma cruzi, Biochemistry (Moscow), 88, 131-141, https://doi.org/10.1134/S000629792301011X.
Acknowledgments
Equipment acquired through the program of Moscow State University Development, FT-IR Bruker Tensor 27 spectrometer (Germany) and CD-spectrometer Jasco J-815 (JASCO, Japan), was used in this work.
Author information
Authors and Affiliations
Contributions
E.V.K. concept of the study and supervision of the work; A.A.Ch. conducting experiments; E.V.K. and A.A.Ch. discussion of the study results; A.A.Ch. writing text of the paper; E.V.K. and A.A.Ch. editing text of the paper.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest in financial or any other sphere. This work does not describe any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chudin, A.A., Kudryashova, E.V. Impact of Lipid Matrix Composition on Activity of Membranotropic Enzymes Galactonolactone Oxidase from Trypanosoma cruzi and L-Galactono-1,4-Lactone Dehydrogenase from Arabidopsis thaliana in the System of Reverse Micelles. Biochemistry Moscow 88, 2073–2083 (2023). https://doi.org/10.1134/S0006297923120106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923120106