Abstract
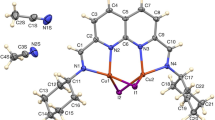
The ligand environment of the \([\{\text{Mo}_{5}\text{S}_{5}^{\text{i}}(\text{pz})_{4}^{\text{i}}\}(\text{pzH})_{5}^{\text{t}}]\text{Br}_{2}\) cluster complex is modified by a reaction of the cluster with Br2 in N,N-dimethylformamide at room temperature, which results in bromination of all pyrazole and pyrazolate ligands at the fourth position and in bromide ligand substitution for apical pyrazole. The solvent effect on ligand bromination is considered. The \([\{\text{Mo}_{5}\text{S}_{5}^{\text{i}}(4\text{-Br-pz})_{4}^{\text{i}}\}(4\text{-Br-pzH})_{4}^{\text{bs}}\text{B}{{\text{r}}^{\text{a}}}]\text{Br}\cdot \text{E}{{\text{t}}_{2}}\text{O}\cdot \text{5DMF}\) cluster complex crystallizes in the tetragonal symmetry (space group P4/n) and forms infinite layers via hydrogen bonds between the terminal 4-bromopyrazole ligands and the bromine anion. The complex also demonstrates reversible one-electron reductions of the cluster core shifted relative to the initial compound by 30 mV and 90 mV towards larger potentials.







Similar content being viewed by others
REFERENCES
V. Fedorov. Metal clusters. As they were born in Siberia. J. Clust. Sci., 2015, 26(1), 3-15. https://doi.org/10.1007/s10876-014-0736-y
Y. M. Gayfulin, Y. V. Mironov, and N. G. Naumov. High-valence cluster compounds of transition metals containning interstitial heteroatoms: Geometry, electronic structure, and physicochemical properties. J. Struct. Chem., 2021, 62(3), 331-355. https://doi.org/10.1134/s002247662103001x
A. L. Gushchin, Y. A. Laricheva, M. N. Sokolov, and R. Llusar. Tri- and tetranuclear molybdenum and tungsten chalcogenide clusters: On the way to new materials and catalysts. Russ. Chem. Rev., 2018, 87(7), 670-706. https://doi.org/10.1070/rcr4800
S. N. Khanna, A. C. Reber, D. Bista, T. Sengupta, and R. Lambert. The superatomic state beyond conventional magic numbers: Ligated metal chalcogenide superatoms. J. Chem. Phys., 2021, 155(12). https://doi.org/10.1063/5.0062582
K. Kirakci, M. A. Shestopalov, and K. Lang. Recent developments on luminescent octahedral transition metal cluster complexes towards biological applications. Coord. Chem. Rev., 2023, 481, 215048. https://doi.org/10.1016/j.ccr.2023.215048
N. T. K. Nguyen, C. Lebastard, M. Wilmet, N. Dumait, A. Renaud, S. Cordier, N. Ohashi, T. Uchikoshi, and F. Grasset. A review on functional nanoarchitectonics nanocomposites based on octahedral metal atom clusters (Nb6, Mo6, Ta6, W6, Re6): inorganic 0D and 2D powders and films. Sci. Technol. Adv. Mater., 2022, 23(1), 547-578. https://doi.org/10.1080/14686996.2022.2119101
M. A. Mikhaylov and M. N. Sokolov. Molybdenum iodides - from obscurity to bright luminescence. Eur. J. Inorg. Chem., 2019, 2019(39/40), 4181-4197. https://doi.org/10.1002/ejic.201900630
S. Akagi, S. Fujii, and N. Kitamura. A study on the redox, spectroscopic, and photophysical characteristics of a series of octahedral hexamolybdenum(II) clusters: [{Mo6X8}Y6]2– (X, Y = Cl, Br, or I). Dalton Trans., 2018, 47(4), 1131-1139. https://doi.org/10.1039/c7dt04485b
D. V. Evtushok, A. R. Melnikov, N. A. Vorotnikova, Y. A. Vorotnikov, A. A. Ryadun, N. V. Kuratieva, K. V. Kozyr, N. R. Obedinskaya, E. I. Kretov, I. N. Novozhilov, Y. V. Mironov, D. V. Stass, O. A. Efremova, and M. A. Shestopalov. A comparative study of optical properties and X-ray induced luminescence of octahedral molybdenum and tungsten cluster complexes. Dalton Trans., 2017, 46(35), 11738-11747. https://doi.org/10.1039/c7dt01919j
A. A. Krasilnikova, A. O. Solovieva, A. A. Ivanov, K. E. Trifonova, T. N. Pozmogova, A. R. Tsygankova, A. I. Smolentsev, E. I. Kretov, D. S. Sergeevichev, M. A. Shestopalov, Y. V. Mironov, A. M. Shestopalov, A. F. Poveshchenko, and L. V. Shestopalova. Comprehensive study of hexarhenium cluster complex Na4[{Re6Te8}(CN)6] - In terms of a new promising luminescent and X-ray contrast agent. Nanomedicine, 2017, 13(2), 755-763. https://doi.org/10.1016/j.nano.2016.10.016
M. V. Shamshurin, M. A. Mikhaylov, T. Sukhikh, E. Benassi, A. R. Tarkova, A. A. Prokhorikhin, E. I. Kretov, M. A. Shestopalov, P. A. Abramov, and M. N. Sokolov. Octahedral {Ta6I12} clusters. Inorg. Chem., 2019, 58(14), 9028-9035. https://doi.org/10.1021/acs.inorgchem.9b00364
A. A. Ulantikov, Y. M. Gayfulin, A. A. Ivanov, T. S. Sukhikh, M. R. Ryzhikov, K. A. Brylev, A. I. Smolentsev, M. A. Shestopalov, and Y. V. Mironov. Soluble molecular rhenium cluster complexes exhibiting multistage terminal ligands reduction. Inorg. Chem., 2020, 59(9), 6460-6470. https://doi.org/10.1021/acs.inorgchem.0c00546
V. K. Muravieva, I. P. Loginov, T. S. Sukhikh, M. R. Ryzhikov, V. V. Yanshole, V. A. Nadolinny, V. Dorcet, S. Cordier, and N. G. Naumov. Synthesis, structure, and spectroscopic study of redox-active heterometallic cluster-based complexes [Re5MoSe8(CN)6]n. Inorg. Chem., 2021, 60(12), 8838-8850. https://doi.org/10.1021/acs.inorgchem.1c00763
I. V. Savina, A. A. Ivanov, D. V. Evtushok, Y. M. Gayfulin, A. Y. Komarovskikh, M. M. Syrokvashin, M. N. Ivanova, I. P. Asanov, I. V. Eltsov, N. V. Kuratieva, Y. V. Mironov, and M. A. Shestopalov. Unusual square pyramidal chalcogenide Mo5 cluster with bridging pyrazolate-ligands. Int. J. Mol. Sci., 2023, 24(4), 3440. https://doi.org/10.3390/ijms24043440
I. V. Savina, A. A. Ivanov, I. V. Eltsov, V. V. Yanshole, N. V. Kuratieva, A. Y. Komarovskikh, M. M. Syrokvashin, and M. A. Shestopalov. Chemical diversity of Mo5S5 clusters with pyrazole: Synthesis, redox and UV-vis-NIR absorption properties. Int. J. Mol. Sci., 2023, 24(18), 13879. https://doi.org/10.3390/ijms241813879
G. M. Sheldrick. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053273314026370
G. M. Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053229614024218
J. D. Vaughan, D. G. Lambert, and V. L. Vaughan. Kinetics and mechanism of iodination of pyrazole. Comparative reactivities of pyrazole and imidazole. J. Am. Chem. Soc., 1964, 86(14), 2857-2861. https://doi.org/10.1021/ja01068a019
J. D. Vaughan and W. A. Smith. Kinetics of iodination of nickel(II)-coordinated pyrazole. J. Am. Chem. Soc., 1972, 94(7), 2460-2463. https://doi.org/10.1021/ja00762a044
H. R. Matthews, L. C. Bucke, and A. G. Blackman. Electrophilic substitution of metal-coordinated pyrazole: Nitration, sulfonation and bromination of [Co(NH3)5(pyzH)]3+ (pyzH = pyrazole). Inorg. Chim. Acta, 1998, 277(1), 89-97. https://doi.org/10.1016/s0020-1693(97)06120-3
K. L. Olsen, M. R. Jensen, and J. A. MacKay. A mild halogenation of pyrazoles using sodium halide salts and oxone. Tetrahedron Lett., 2017, 58(43), 4111-4114. https://doi.org/10.1016/j.tetlet.2017.09.042
Z. Zhao and Z. Wang. Halogenation of pyrazoles using N-halosuccinimides in CCl4 and in water. Synth. Commun., 2007, 37(1), 137-147. https://doi.org/10.1080/00397910600978549
Y. L. Janin. Preparation and chemistry of 3/5-halogenopyrazoles. Chem. Rev., 2012, 112(7), 3924-3958. https://doi.org/10.1021/cr200427q
S. Guillou, F. J. Bonhomme, M. S. Ermolenko, and Y. L. Janin. Simple preparations of 4 and 5-iodinated pyrazoles as useful building blocks. Tetrahedron, 2011, 67(44), 8451-8457. https://doi.org/10.1016/j.tet.2011.09.029
Funding
The work was supported by the Russian Science Foundation (Grant No. 19-73-20109).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 12, 122349.https://doi.org/10.26902/JSC_id122349
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Savina, I.V., Ivanov, A.A. & Shestopalov, M.A. Bromination of Pyrazole and Pyrazolate Ligands in [Mo5S5(pz)4(pzH)5]Br2. J Struct Chem 64, 2451–2460 (2023). https://doi.org/10.1134/S0022476623120168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623120168