Abstract
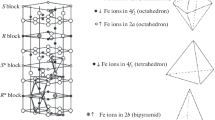
Distribution of chemical elements in polycrystalline BaFe12–xTixO19 and BaFe12–xMnxO19 barium ferrite samples is studied. The samples are prepared by solid-phase synthesis at 1400 °C from stoichiometric mixtures of oxides and carbonates. The XRD data indicate that all the studied samples have one crystalline phase characteristic of the M-type hexaferrite structure. The Curie temperatures are determined by differential scanning calorimetry. It is shown that replacing iron with Ti and Mn atoms diminishes the temperature of magnetic phase transition. The difference in bulk and surface atomic composition between the studied ferrites are established by XRD and XPS. It is shown that barium can exhibit surface segregation. The replacement of iron by manganese in the barium hexaferrite structure leads to surface segregation of barium, while the replacement by titanium hinders the segregation.







Similar content being viewed by others
REFERENCES
Z. F. Abdurashidova and N. A. Nurmatov. Izuchenie vliyaniya segregatsii i termodiffuzii atomov bariya na fotoemissionnuyu effektivnost′ splava Mg–Ba (1%) (Study of the influence of segregation and thermal diffusion of barium atoms on the photoemissive efficiency of the alloy Mg–Ba (1%)). Akad. Issled. Obl. Pedag. Nauk, 2021, 2(4), 677-682. [In Russian]
V. G. Shevchenko, M. V. Kuznetsov, S. A. Bibanaeva, A. V. Konyukova, I. A. Chupova, I. N. Latosh, V. A. Kochedykov, and D. A. Eselevich. Poverhnostnaya segregaciya kal′ciya i ee vliyanie na kinetiku okisleniya poroshkov splavov na osnove alyuminiya (Surface segregation of calcium and its influence on the kinetics of oxidation of aluminum-based alloy powders). Fizikokhim. Poverkhn. Zashch. Mater., 2012, 48(6), 540-545. [In Russian]
V. G. Shevchenko, D. A. Eselevich, A. I. Ancharov, and B. P. Tolochko. Vliyanie bariya na kinetiku okisleniya poroshka splava na osnove alyuminiya (Effect of barium on the oxidation kinetics of aluminum-based alloy powder). Fiz. Goreniya Vzryva, 2014, 50(6), 28-33. [In Russian]
A. S. Li. Sposob izgotovleniya oksidnogo katoda (Method for manufacturing an oxide cathode). Patent RU 2 060 570 C1, 1995. [In Russian]
L. Wang, J. Zhang, Q. Zhang, N. Xu, and J. Song. XAFS and XPS studies on site occupation of Sm3+ ions in Sm doped M-type BaFe12O19. J. Magn. Magn. Mater., 2015, 377, 362-367. https://doi.org/10.1016/J.JMMM.2014.10.097
K.-I. Mehnert, M. Häßner, Y. M. Dreer, I. Biswas, and R. Niewa. Crystal structure and XPS study of titanium-substituted M-type hexaferrite BaFe12–xTixO19. Inorganics, 2023, 11(5), 207. https://doi.org/10.3390/inorganics11050207
M. Saeedi, M. Abdellahi, A. Rahimi, and A. Khandan. Preparation and characterization of nanocrystalline barium ferrite ceramic. Funct. Mater. Lett., 2016, 09(05), 1650068. https://doi.org/10.1142/s1793604716500685
R. Pattanayak, S. Panigrahi, T. Dash, R. Mudulisurname, and D. Beherasurname. Electric transport properties study of bulk BaFe12O19 by complex impedance spectroscopy. Phys. B, 2015, 474, 57-63. https://doi.org/10.1016/J.PHYSB.2015.06.006.
K. Huang, J. Yu, L. Zhang, J. Xu, Z. Yang, C. Liu, W. Wang, and X. Kan. Structural and magnetic properties of Gd–Zn substituted M-type Ba–Sr hexaferrites by sol-gel auto-combustion method. J. Alloys Compd., 2019, 803, 971-980. https://doi.org/10.1016/J.JALLCOM.2019.06.348.
K. Huang, J. Yu, L. Zhang, J. Xu, P. Li, Z. Yang, C. Liu, W. Wang, and X. Kan. Synthesis and characterizations of magnesium and titanium doped M-type barium calcium hexaferrites by a solid state reaction method. J. Alloys Compd., 2020, 825, 154072. https://doi.org/10.1016/J.JALLCOM.2020.154072.
A. Bhaduri, S. Singh, K. B. Thapa, and B. C. Yadav. Visible light-induced, highly responsive, below lower explosive limit (LEL) LPG sensor based on hydrothermally synthesized barium hexaferrite nanorods. Sens. Actuators B, 2021, 348, 130714. https://doi.org/10.1016/J.SNB.2021.130714
S. Anand, S. Pauline, and C. J. Prabagar. Zr doped Barium hexaferrite nanoplatelets and RGO fillers embedded polyvinylidenefluoride composite films for electromagnetic interference shielding applications. Polym. Test., 2020, 86, 106504. https://doi.org/10.1016/J.POLYMERTESTING.2020.106504
M. Manikandan and C. Venkateswaran. Effect of high energy milling on the synthesis temperature, magnetic and electrical properties of barium hexagonal ferrite. J. Magn. Magn. Mater., 2014, 358/359, 82-86. https://doi.org/10.1016/j.jmmm.2014.01.041
V. V. Atuchin, D. A. Vinnik, T. A. Gavrilova, S. A. Gudkova, L. I. Isaenko, X. Jiang, L. D. Pokrovsky, I. P. Prosvirin, L. S. Mashkovtseva, and Z. Lin. Flux crystal growth and the electronic structure of BaFe12O19 hexaferrite. J. Phys. Chem. C, 2016, 120, 5114-5123. https://doi.org/10.1021/acs.jpcc.5b12243
D. A. Vinnik, D. A. Zherebtsov, and L. S. Mashkovceva. Vyrashchivanie legirovannyh monokristallov ferrita bariya iz flyusa (Growing doped barium ferrite single crystals from flux). Dokl. Akad. Nauk, 2013, 449, 174/175. https://doi.org/10.7868/S0869565213080161 [In Russian]
K. V. Chernyakova, V. V. Pan′kov, M. I. Ivanovskaya, and V. A. Lomonosov. Struktura i magnitnye svojstva geksagonal′nogo ferrita bariya (Structure and magnetic properties of hexagonal barium ferrite). Vestn. BGU, 2008, 2(1), 9-13. [In Russian]
D. A. Vinnik, F. V. Podgornov, N. S. Zabeivorota, E. A. Trofimov, V. E. Zhivulin, A. S. Chernukha, M. V. Gavrilyak, S. A. Gudkova, D. A. Zherebtsov, A. V. Ryabov, S. V. Trukhanov, T. I. Zubar, L. V. Panina, S. V. Podgornaya, M. V. Zdorovets, and A. V. Trukhanov. Effect of treatment conditions on structure and magnetodielectric properties of barium hexaferrites. J. Magn. Magn. Mater., 2020, 498, 166190. https://doi.org/10.1016/j.jmmm.2019.166190
V. E. Zhivulin, I. A. Solizoda, D. A. Vinnik, S. A. Gudkova, E. A. Trofimov, A. Y. Starikov, O. V. Zaitseva, D. P. Sherstyuk, A. E. Vasiljeva, D. A. Zherebtsov, S. V. Taskaev, P. A. Zezyulina, D. A. Petrov, and A. V. Trukhanov. Impact of Al3+ ions on magnetic and microwave properties of BaM:Ti hexaferrites. J. Mater. Res. Technol., 2021, 11, 2235-2245. https://doi.org/https://doi.org/10.1016/j.jmrt.2021.02.051.
P. A. Zezyulina, D. A. Petrov, K. N. Rozanov, D. A. Vinnik, S. S. Maklakov, V. E. Zhivulin, A. Y. Starikov, D. P. Sherstyuk, and S. Shannigrahi. Study of the static and microwave magnetic properties of nanostructured BaFe12−xTixO19. Coatings, 2020, 10(8), 789. https://doi.org/10.3390/coatings10080789
X. Obradors, A. Collomb, M. Pernet, J. C. Joubert, and A. Isalgué, Structural and magnetic properties of BaFe12–xMnxO19 hexagonal ferrites. J. Magn. Magn. Mater., 1984, 44, 118-128. https://doi.org/10.1016/0304-8853(84)90053-2
D. A. Vinnik. Poluchenie monokristallov geksaferrita bariya svinca iz rastvora (Preparation of lead barium hexaferrite single crystals from solution). Vestn. Yuzhno-Ural. Gos. Univ., Ser. Metall., 2016. 16(1), 7-12. https://doi.org/10.14529/met160101 [In Russian]
A. M. Lebedev, K. A. Menshikov, V. G. Nazin, V. G. Stankevich, M. B. Tsetlin, and R. G. Chumakov. NanoPES photoelectron beamline of the kurchatov synchrotron radiation source. J. Surf. Invest.: X-Ray, Synchrotron Neutron Tech., 2021, 15, 1039-1044. https://doi.org/10.1134/S1027451021050335
X. Obradors, A. Collomb, M. Pernet, D. Samaras, and J. C. Joubert. X-ray analysis of the structural and dynamic properties of BaFe12O19 hexagonal ferrite at room temperature. J. Solid State Chem., 1985, 56, 171-181. https://doi.org/10.1016/0022-4596(85)90054-4
D. A. Shirley. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B., 1972, 5, 4709-4714. https://doi.org/10.1103/PhysRevB.5.4709
CasaXPS, 2.3.25. Teignmouth, U.K.: Casa Software, 2022.
S. Tanuma, C. Powell, and D. Penn. Inelastic mean free paths of low energy electrons in solids. Acta Phys. Pol., A, 1992, 81(2), 169-186, https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=620392 (accessed Aug 17, 2023).
Funding
This study was funded by the Ministry of Science and Higher Education of the Russian Federation as part of Agreement No. 075-15-2021-1351.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 12, 119470.https://doi.org/10.26902/JSC_id119470
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pesin, L.A., Gudkova, S.A., Zhivulin, V.E. et al. Effect of Titanium and Manganese Additions on the Surface Segregation of Barium in Hexaferrites. J Struct Chem 64, 2358–2369 (2023). https://doi.org/10.1134/S0022476623120077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623120077