Abstract
Sepsis has a systemic inflammatory response syndrome caused by infection. While neutrophils play contradictory roles in different stages of sepsis. Neutrophils have been proven to play an antibacterial role by producing neutrophil extracellular traps (NETs). Although the NET is beneficial to bacteria resistance, abnormal NET increases tissue damage. The complement C5a receptor 1 (C5ar1) is a gene related to strong inflammatory reactions and is found to be associated with inflammatory factors. This study found that there were 45 down-regulated genes and 704 up-regulated genes in sepsis rats by transcriptome sequencing. And those genes were significantly related to inflammation and immunity by GO and KEGG enrichment analysis involving the chemokine signaling pathway, the Toll-like receptor (TLR) signaling pathway, and the Fc gamma R-mediated phagocytosis. Additionally, the C5ar1 gene was significantly upregulated with interesting potential in sepsis and used for further study. This study used cecum ligation and puncture (CLP) rats that were respectively injected intravenously with PBS or the lentivirus vector to explore the effect of C5ar1 on CLP rats. It demonstrated that silenced- C5ar1 inhibited the ALT, AST, BUN, and CREA levels, improved the lung and spleen injury, and reduced the TNF-α, IL-6, IL-1β, IL-10, cf-DNA, and cfDNA/MPO levels. Additionally, silenced C5ar1 inhibited the TLR2, TLR4, and peptidylarginine deiminase 4 expression levels, which suggested the improvement of silenced C5ar1 on sepsis via inhibiting NETs and the TLR signaling pathway. This study provides a basis and new direction for the study of treatment on sepsis.
Similar content being viewed by others
Introduction
According to a Global Burden of Diseases analysis, the sepsis mortality rate reduced by 52.8% between 1990 and 2017 as a result of the development of multiple organ support and other treatments, however, it is anticipated that sepsis mortality rate will still account for 19.7% of the global mortality (Rudd et al. 2020). Over the past two decades, timely use of antibiotics, fluid resuscitation, and multiple organ support therapies have gradually reduced sepsis mortality. But there is still sizable mortality and space for improvement. Sepsis, with high morbidity and mortality, has a systemic inflammatory response syndrome caused by infection (Du et al. 2022). Multiple organ dysfunction syndromes are characteristic in the later stage of sepsis and it’s hard to treat once it happens (Middleton et al. 2019). And in the innate immune system of sepsis, most congenital cells tend to experience apoptosis, such as dendritic cells, immature macrophages, and natural killer cells, while neutrophils show delayed apoptosis, which may be related to organ failure in late sepsis (Kovach and Standiford 2012). Ding et al. reported lung damage with neutrophil recruitment in sepsis rats (Ding et al. 2021). Neutrophils have been proven to play an antibacterial role by producing neutrophil extracellular traps (NETs), and NET formation is a process called NETosis (Tan et al. 2021). A study reported that although NET benefits bacteria resistance, abnormal NETs increased tissue damage (Colón et al. 2019). And degradation of NETs combined with antibiotic treatment can significantly improve the survival rate of septic mice (Czaikoski et al. 2016). Specifically, researchers found that degradation of NETs at 6 h after CLP improved organ damage (Mai et al. 2015). NETs were composed of extracellular chromatin decorated with histones and numerous granular proteins including myeloperoxidase (MPO) and elastase (NE) (Brinkmann and Zychlinsky 2007). Therefore, sepsis prevention and treatment need to explore the regulation of NETs in neutrophils. Although neutrophilic NETs are important in the treatment of sepsis, the research on their biological mechanisms is still insufficient.
In recent years, the technology of sequencing has been widely used in pathological and pharmacological studies of many diseases. New disease targets have been discovered through transcriptomics technology, and the new mechanisms will provide a basis and new direction for the study of disease diagnosis and treatment (Prokop et al. 2018). The potential diagnostic markers and therapeutic targets can be found through transcriptome sequencing to provide more exploration directions for disease diagnosis and treatment (Saeidian et al. 2020). Next-generation sequencing has become a first-line tool for the diagnosis of primary immunodeficiencies (Platt et al. 2021). And Riazuddin and co-workers identified 30 novel candidate genes for recessive intellectual disability by sequencing (Riazuddin et al. 2017). Especially, Middleton and co-workers used parallel techniques of RNA sequencing and ribosome footprint profiling to interrogate the platelet transcriptome and translatome in septic patients and healthy donors (Middleton et al. 2019). Therefore, this study explored the gene expression between sepsis rats with its control by transcriptome sequencing. And this study noticed the high expression of complement C5a receptor 1 (C5ar1).
C5ar1 is a gene related to strong inflammatory reactions and is found to be associated with interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)-α, and other inflammatory factors (Shi et al. 2021). A study found that inhibition of C5ar1 can antagonize NETs-induced thrombosis in vitro (Skendros et al. 2020). C5ar1 mice are more resistant to invasive meningococcal infection than wild-type mice, however, it is interesting that the pharmacological blockade of C5ar1 improves the survival rate of mice after sepsis induction (Herrmann et al. 2018). Additionally, in cancer cells, C5a induced the formation of ENTs in myeloid-derived suppressor cells (Ortiz-Espinosa et al. 2022). Because the biological mechanism of C5ar1 and ENTs of sepsis is still unclear, this study established an animal model of sepsis through cecal ligation and puncture (CLP) in rats, to explore the function of C5ar1, providing a basis and new direction for the study of diagnosis and treatment on sepsis patients.
Materials and methods
Construction of sepsis animal model
The sepsis animal model was constructed using the Sprague Dawley (SD) (200–230 g) rats with 7 days of adaptive feeding by cecum ligation and puncture (CLP) under specific pathogen-free conditions in the animal facility. The 36 SD rats were purchased from Shanghai Jihui Laboratory Animal Care Co. All procedures on animals are approved by the Animal Experimentation Ethics Committee of Zhejiang Eyong Pharmaceutical Research and Development Center (SYXK (Zhe)2021-0033). The isoflurane-anesthetized rats were made incisions in the middle of the abdominal wall to ligate at 1/3 from the end of the cecum by using the sterile No. 4 thread. Subsequently, a 21G injection needle was punctured about 3 to 4 times at the middle of the ligature site to the top of the caecum. The rats were sutured and resuscitated with 5 mL/100 g of normal saline. The sham rats underwent surgery like the CLP rats except for the operation of ligation and puncture. At 24 h after the CLP, whole blood was taken from 6 sham rats and 6 CLP rats and stored in the tube containing EDTA (BD Vacutainer, Franklin Lakes, NJ, USA) for RNA-seq. Then all rats were respectively injected intravenously with the PBS or the lentivirus vector (about 2 × 107 transforming units) that contained the C5ar1 gene or the empty lentivirus vector. SD rats were divided into 4 groups (n = 6), the sham, the CLP, the sh-NC, and the sh-Serpinb1a groups. At 48 h after injection, blood was taken from rats of each group and was centrifugated for serum. After rats were euthanized, the lung and spleen were taken out and divided into two parts, one was fixed and embedded in paraffin, and the other was stored at − 80 °C.
Transcriptome sequencing
The fresh blood samples were sent to LianChuang Biomedical Tech Co., Ltd, Hangzhou, China. The transcriptome sequencing was performed as Liao’s team reported (Liao et al. 2021). After quality evaluation of the total RNA by NanoDrop ND-1000 (NanoDrop, Wilmington, DE, USA), was detected by Bioanalyzer 2100 (Agilent, CA, USA). The mRNA was fragmented after specific capture by Dynabeads Oligo (25-61005, Thermo Fisher, USA). After normalizing the PCR libraries, the High throughput sequencing was started by Illumina Novaseq™ 6000 (LC Bio-Technology, China). After quality control, the raw data file (FASTQ) is used for subsequent analysis of differences and correlations. Log 2 (fold change) ≥ 1 was the gene threshold. The P-value < 0.05 is the standard for screening differential genes. Then Gene Ontology (Go) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were additionally performed on the genes.
Histological analysis
The 5 µM paraffin-embedded sections of the lung and spleen were performed as described previously by Hematoxylin-eosin staining (HE) (Bry-001, Runnerbio, China) (Sun et al. 2021). All evaluations are semi-quantitative scores and are performed by professionals who were blinded to group allocation. The semi-quantitative assessment of pulmonary injury was scored on alveolar edema, alveolar septal thickening, and inflammatory cell infiltration (Wen et al. 2020; Yang et al. 2021). And spleen injury was scored on histomorphology and the area of red pulp and white pulp, and edema (Zhang et al. 2019). Semi-quantitative scores were determined by two independent observers unaware in a blind fashion.
Quantitative determination of cell free-DNA (cf-DNA) and the cf-DNA bound to MPO (cf-DNA/MPO)
To detect NETs, the cf-DNA and cf-DNA/MPO levels were measured as previously described (Czaikoski et al. 2016). The PicoGreen dsDNA kit (P7589, Invitrogen, USA) was used to measure the level of the cf-DNA in serum. In brief, at room temperature, the serum diluted with PBS (1:10) and the fluorescent reagent were mixed in a 96-well plate and the absorbance were detected by the plate reader at the wavelength of 485/538 nm. The cf-DNA concentration was calculated according to the standard curve. In addition, the concentration of cf-DNA/MPO was measured in the 96-well plate that coated with anti-MPO monoclonal antibody (ab208670, Abcam, UK) using the PicoGreen dsDNA kit as above.
Inflammatory cytokines level analysis
The inflammatory cytokines were measured by enzyme-linked immunosorbent assays (ELISA) according to the instructions. The interleukin 6 (IL-6) (MM-0190R2, MEIMIAN, China), IL-10 (PI525, Beyotime, China), IL-1β (MM-0099R2, MEIMIAN, China), and TNF-α (MM-0180R2, MEIMIAN, China) were used for evaluating the level of inflammation in serum. The OD was measured at 450 nm by a microplate reader (CMaxPlus, Molecular Devices, USA). Their concentration was directly proportional to the OD (450 nm) value, which was calculated by plotting a standard curve.
Semi-quantitative analysis of protein levels
Western blot was used to perform the semi-quantitative analysis of the proteins in the neutrophils. Neutrophils were isolated from rat blood using Percoll solution (P8370, Solarbio, China) by density gradient centrifugation (30 min at 500×g) as previously reported (Xue et al. 2017). Neutrophils were resuspended in RPMI-1640 containing 5% FBS. Then after lysing of the neutrophils by RIPA lysis buffer (P0013E, Beyotime, China) with protease and phosphatase inhibitors (P0013C, Beyotime, China), the quantitative analysis of total protein levels was measured using a BCA kit (4240GR100, Beyotime, China) in 96-well plate. The denatured proteins were separated in SDS-PAGE electrophoresis, transferred to the PVDF membrane, and incubated with 5% fat-free milk. Subsequently, the blocked membranes were handled at 4 °C with anti-TLR2 (1:2000, 17236-1-AP, Proteintech, USA), anti-TLR4 (1:2000, AF7017, Affinity, USA), anti-peptidylarginine deiminase 4 (PAD4) (1:5000, DF6685, Affinity, USA) and anti-C5ar1 (1:5000, DF10210, Affinity, USA), and anti-GAPDH (1:30000, AF7021, Affinity, USA) antibodies overnight and further incubated with corresponding secondary antibody (1:5000, CST, USA) at RT for 1.5 h. Finally, the membrane was incubated with ECL reagents (610020-9Q, Qing Xiang, China) and visualized by ImageJ software.
Statistical analysis
The multiple group’s data with normal distribution and the homogeneous variance were analyzed by the One-way-ANOAY following the Tukey test. And the Kruskal–Wallis H test was used to analyze the data without the normal distribution. All data were analyzed by SPSS 26.0. and expressed as mean (standard deviation), P < 0.05 means the difference was statistically significant.
Results
Screening and analysis of potential target genes
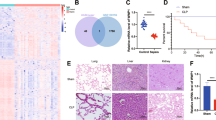
Comparative analysis of CLP and sham rat genes by transcriptome sequencing. The expression trend of biological repeated samples tends to be consistent (Fig. 1a). And the volcanic map shows the overall distribution of differentially expressed genes, there are a total of 32,623 genes, 704 up-regulated genes, and 45 down-regulated genes (Fig. 1b). The Heatmap was generated by cluster analysis of the Z value for all significantly different genes (Supplementary Fig. 1) and showed the top 100. Additionally, we performed the PPI network construction and analysis for all significantly upregulated genes by STRING. The PPI was modified by the Cytoscape to remove neighbor-free nodes (Supplementary Fig. 2). An average number of node degree is 9.025, which means there are relevant number of neighbors. There are 189 genes with number of neighbors greater than 9.025. The Supplemental Table 1 lists 183 gene with neighbor degrees greater than average that can find the ENSEMB ID in DAVID database, including Fn1 (degree = 67), Tlr4 (degree = 64), Mmp9 (degree = 62), Tyrobp (degree = 62), Tlr2 (degree = 58), Itgam (degree = 54), Vamp8 (degree = 49), Casp3 (degree = 47), C5ar1 (degree = 46), et al. The expression of top 30 of these 183 genes were shown in Fig. 2.
Gene expression value density and differentially expressed genes overall distribution. a The expression value density diagram. b The volcano of scatters plots. The red represents significantly up-regulated genes, blue represents significantly down-regulated genes, and gray represents the gene expression without significant differential
Enrichment analysis
Additionally, the results of the GO enrichment analysis of 704 up-regulated genes showed that the inflammatory response, the immune system process, the innate immune response, and the neutrophil chemotaxis were the top 4 terms with the lowest P-value (Fig. 3a and d) showed the statistics of KEGG pathway enrichment and most of those pathways are involved in immunity and inflammation, such as the chemokine signaling pathway, the Toll-like receptor signaling pathway, and the Fc gamma R-mediated phagocytosis.
The enrichment analysis of Protein-Protein Interaction (PPI) networks. a The bubble chart of Gene Ontology (GO) enrichment for all potential target genes. There are shown the top 20, and the rich factor means that in the GO term, the ratio of the number of differential genes located to the number of total genes located. b The bubble chart of KEGG enrichment for all potential target genes. There are shown the top 20 pathways with the lowest P-value. The Rich Factor indicates the ratio of the potential target gene number in the pathway to the total gene number in the pathway, the value of the Rich Factor is proportional to the enrichment degree
Accordingly, we observed the expression levels of top 10 genes in Supplemental Tables 1 and found the expression levels of Fn1, Tyrobp, Vamp8, and C5ar1 were enormously increased in CLP rats (Fig. 4a). We have noticed that C5ar1 related proteins include TLR2, TLR4, Casp3, etc. (Fig. 4b). The 47 genes that related to C5ar1 were enriched and analyzed using GO and KEGG enrichment analysis function on the GENE DENOVO platform (http://www.omicshare.com), and the results are shown in Fig. 4c, d. They were mainly involved in the Osteoclast differentiation, the Staphylococcus aureus infection, the Tuberculosis, the Phagosome, the Toll-like receptor signaling pathway, and the Cytokine-cytokine receptor interaction pathways (Fig. 4c). Especially, the main biological processes involved include immune system process, response to stimulus and multi-organism process (Fig. 4d).
Enrichment analysis of complement C5a receptor 1 (C5ar1) and its neighbors. a The expression levels of Fn1, Tlr4, Mmp9, Tyrobp, Tlr2, Itgam, Vamp8, Casp3, C5ar1, and Icam1 in CLP rats. b Protein–Protein Interaction (PPI) networks of C5ar1 and its neighbors. c The bar plot of top 30 pathways from KEGG enrichment for C5ar1 and its neighbors. d The bar plot of the top 30 Gene Ontology (GO) terms in the class of biological process, cellular component, and molecular function. The GO enrichment analysis was performed on C5ar1 and its neighbors
The amelioration of silenced-C5ar1 in CLP rats on liver, kidney, lung, and spleen injury
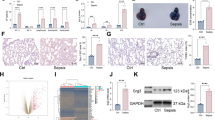
ALT, AST, BUN, and CREA, as biochemical indexes, were used to evaluate the severity of liver and kidney (Fig. 5a–d). The values of ALT, AST, BUN, and CREA were dramatically increased in CLP rats (P <0.01). Additionally, silenced-C5ar1 markedly inhibited the ALT, AST, BUN, and CREA levels (P <0.05 and P <0.01) (Fig. 5a–d).
Blood biochemical index of sepsis rats. a ALT and b AST indicate the degree of liver damage. c BUN and d CREA reflect the degree of renal injury. Data are presented as mean (standard deviation), n = 5. **P <0.01, compared to the sham group; +P <0.05 and ++P <0.01, compared to the sh-NC group. ALT alanine aminotransferase, AST aspartate aminotransferase, BUN blood urea nitrogen, CREA creatinine
The HE staining was used to evaluate the lung and spleen injury degree (Fig. 6). In the sham group, the lungs had complete alveolar structure without neutrophil infiltration or edema, while in the CLP and sh-NC groups, the lungs had thickened alveolar wall, damaged alveolar structure, and infiltrated inflammatory cells which were improved by silenced-C5ar1 (Fig. 6a). In sham rats, the spleen had a complete structure and a clear dividing line between the red and white medulla, while the dividing line was blurred, the white medulla area was expanded, and the red medulla was shrunk in the CLP and sh-NC groups (Fig. 6b). And in the sh-C5ar1 group, the dividing line between the red and white medulla was clearer compared to the sh-NC group (Fig. 6b). The lung and spleen semi-quantitative scores of the CLP group were dramatically larger than that of the sham group (P <0.01) (Fig. 6c, d), while their semi-quantitative scores were decreased by silenced-C5ar1 treatment (P <0.05) (Fig. 6c, d).
Histological damage in sepsis rats by using Hematoxylin-eosin staining. a The representative images of the lung. b The representative images of the spleen. And the semi-quantitative scoring of the lung c and d by professionals who were blinded to group allocation. Magnification, ×100 and ×400 and scale bar = 50 μm; data are presented as mean (standard deviation), n = 5. **P <0.01, compared to the sham group; +P <0.05, compared to the sh-NC group
The amelioration of silenced-C5ar1 in CLP rats on inflammatory cytokines and NET
The ELISA was used to measure the TNF-α, IL-1β, IL-6, and IL-10 levels. Those levels were increased in the CLP rats, while they were significantly decreased in the silenced-C5ar1 rats in comparison with the sh-NC rats (P <0.01) (Fig. 7a–d). The cf-DNA and cf-DNA/MPO is major constituents of NET (Czaikoski et al. 2016). As shown in Fig. 7e, f, the cf-DNA and cf-DNA/MPO were huge increased in CLP rats compared to sham rats (P <0.01), while they were dramatically inhibited in silenced-C5ar1 treatment on CLP rats (P <0.01).
The inflammatory cytokine, cf-DNA and cf-DNA/MPO levels in serum of CLP rats. The a TNF-α, b IL-6, c IL-1β, and d IL-10 levels were measured by enzyme-linked immunosorbent assays. The e cf-DNA and f cf-DNA/MPO were measured by a PicoGreen dsDNA kit to observe the NETs levels. Data are presented as mean (standard deviation), n = 5. **P <0.01, compared to the sham group; ++P <0.01, compared to the sh-NC group. Interleukin IL-6, TNF tumor necrosis factor, cf-DNA cell free
The levels of TLR2, TLR4, PAD4, and C5ar1 in neutrophils of CLP rats
The Western Blot was used to measure the TLR2, TLR4, PAD4, and C5ar1 levels in neutrophils (Fig. 8a). Semi-quantitative analysis of the protein bands revealed the TLR2, TLR4, PAD4, and C5ar1 levels were significantly increased in CLP rats with the sham rats as control (P < 0.05 and P < 0.01), in the contrary, the TLR2, TLR4, PAD4, and C5ar1 levels were significantly decreased in silenced-C5ar1 treatment on CLP rats (P < 0.01) (Fig. 8b–e).
The expression levels of TLR2, TLR4, PAD4, and C5ar1 of peripheral blood neutrophils in CLP rats. a The protein bands of TLR2, TLR4, PAD4, and C5ar1; b–e the results of relative expression analysis. Data are presented as mean (standard deviation), n = 3. *P <0.05 and **P <0.01, compared to the sham group; ++P <0.01, compared to the sh-NC group. TLR toll-like receptor, PAD4 peptidylarginine deiminase 4, C5ar1 complement C5a receptor 1
Discussion
In this study, the gene different between CLP rats and control rats were observed by transcriptome sequencing. There are a total of 32,623 genes, 704 up-regulated genes, and 45 down-regulated genes. Inflammation - and immunity related GO terms had the most significant differences among the results of GO enrichment for all differential genes, in which C5ar1 were related to the inflammatory response, Response to lipopolysaccharide, apoptotic process, and neutrophil chemotaxis. Additionally, this study found that silenced C5ar1 could improve tissue injury, inhibit inflammation. After CLP 24 h, detecting the ALT, AST, BUN, and CREA levels, this study found that rats had injury of liver and kidney function, also, the HE staining observed the damage of lung and spleen in CLP rats. Interestingly, silenced-C5ar1 could improve those damages suggesting high expression of C5ar1 play a crucial role in the damages of tissue in sepsis. This may be related to the regulate of inflammatory factors and anti-inflammatory cytokines after silenced C5ar1. In this study, the IL-6, TNF-α, IL-1β, and IL-10 levels were all reduced. It is well known that increased IL-6 levels in sepsis patients are associated with increased mortality, and a study reported that decreasing IL-6 levels can reduce organ failure in sepsis patients (Liu et al. 2021; Panacek et al. 2004). Also, a study reported that a lower IL-6 level was beneficial to sepsis (Riedemann et al. 2003). Furthermore, in the late stage of sepsis, the anti-inflammatory reaction is dominant, and the increase of IL-10 level can predict the mortality of severe sepsis (Monneret et al. 2004). To sum up, this study demonstrated that silenced-C5ar1 could improve the tissue damage and inflammatory homeostasis of septic rats.
Studies have shown that C5 inhibitors are anti-inflammatory in COVID-19 critically ill patients and decreased neutrophil counts and attenuated NET release (Mastellos et al. 2020). Furthermore, studies have found that sepsis has complex proinflammatory and anti-inflammatory responses, and the destruction of immune balance leads to organ dysfunction and lethality (Delano and Ward 2016). Especially, neutrophils are the key cells of the body against bacterial infection and have a strong antibacterial effect. Study reported that although neutrophils are very important to eliminate bacteria in sepsis, their excessive infiltration will also promote organ failure in the late stage of sepsis. Persistent recruitment of neutrophils and delayed apoptosis may be the main cause of organ failure (Bhan et al. 2016). NET, as the key mechanism of neutrophil antibacterial (Janicova and Relja 2021), scientists have found that its dysregulation increases sepsis associated liver tissue damage(Bukong et al. 2018). It suggests that homeostasis of NET is very important to improve the level of sepsis tissue damage and inflammation. Modern research showed that generation of C5a is association with inflammation in sepsis (Zetoune and Ward 2020). Silencing C5aR1 increased survival of CLP mice in sepsis and decreased the secretion of cytokines (Muenstermann et al. 2019; Kalbitz et al. 2016). Additionally, a study reported that C5a induced the change in polarization of neutrophils (Denk et al. 2017). Polarization of neutrophils to the N2 type exhibits a longer lifespan and releases IL-6 and NET to promote cancer development (Wang et al. 2018). Moreover, C5aR1-knock-down animals has a lower MPO level in lung of mice with polytrauma and hemorrhagic shock (Chakraborty et al. 2021). Additionally, the PAD4 is a crux of the NET formation (Hawez et al. 2022). This study found silenced-C5ar1 inhibited the PAD4 level in neutrophils. Although C5a activation of neutrophils has been shown, association of C5ar1 with NETs is less clear. This study investigated the relationship between C5ar1 and NET levels in neutrophils from sepsis rat by silencing C5ar1 in CLP rats and demonstrated the effect of silenced-C5ar1 to reduce NET levels.
In this study, 189 potential key genes were screened, of which the top 10 genes were related to inflammation and anti-inflammatory reaction. Additionally, the KEGG enrichment analysis of C5ar1 and its related genes showed that Toll-like receptor signaling pathway may be related to these genes. Moreover, this study predicted interactions of TLR2, TLR4 and C5ar1. Research have shown that NET can promote the differentiation directly via TLR2 (Wilson et al. 2022) suggesting silenced-C5ar1 inhibited NET to reduce the Tlr2 level. While song et al. reported that extracellular vesicles can promote CLEC5A and TLR2 levels of neutrophils and macrophages, thereby induce NET formation (Sung et al. 2019). This study shown that silenced-C5ar1 inhibited the degree of NET and TLR2 suggesting silenced-C5ar1 may improve the sepsis via the Toll-like receptor signaling pathway. The causal relationship between them needs to be further verified. This study provided a new idea and research direction for the treatment of sepsis.
In conclusion, through transcriptome sequencing, this study reported that there were 704 up-regulated genes and 45 down-regulated genes in CLP rats compared to sham rats. Enrichment analysis showed that these genes are involved in immune, inflammatory, and infection-elated response and signaling pathways. Moreover, this study found C5ar1 has a high expression in CLP rats and predicted that C5ar1 may be related with the TLR signaling pathway. Additionally, this study found silenced C5ar1 in CLP rats inhibited the levels of ALT, AST, BUN, CREA, TNF-α, IL-6, IL-1β, IL-10, cf-DNA, and cf-DNA/MPO in serum, and decreased the expression levels of TLR2, TLR4, and PAD4 proteins in neutrophils. Moreover, silenced-C5ar1 improved lung and spleen damage of CLP rats. Summing up, this study suggested that inhibiting the C5ar1 level can inhibit over-expression ENTs to balance the inflammatory levels in CLP rats and improve the tissue damage in sepsis, providing a basis and new direction for the study of treatment on sepsis patients.
References
Bhan C, Dipankar P, Chakraborty P, Sarangi PP (2016) Role of cellular events in the pathophysiology of sepsis. Inflamm Res 65(11):853–868. https://doi.org/10.1007/s00011-016-0970-x
Brinkmann V, Zychlinsky A (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5(8):577–582. https://doi.org/10.1038/nrmicro1710
Bukong TN, Cho Y, Iracheta-Vellve A, Saha B, Lowe P, Adejumo A, Furi I, Ambade A, Gyongyosi B, Catalano D, Kodys K, Szabo G (2018) Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J Hepatol 69(5):1145–1154. https://doi.org/10.1016/j.jhep.2018.07.005
Chakraborty S, Winkelmann VE, Braumüller S, Palmer A, Schultze A, Klohs B, Ignatius A, Vater A, Fauler M, Frick M, Huber-Lang M (2021) Role of the C5a–C5a receptor axis in the inflammatory responses of the lungs after experimental polytrauma and hemorrhagic shock. Sci Rep 11(1):2158. https://doi.org/10.1038/s41598-020-79607-1
Colón DF, Wanderley CW, Franchin M, Silva CM, Hiroki CH, Castanheira FVS, Donate PB, Lopes AH, Volpon LC, Kavaguti SK, Borges VF, Speck-Hernandez CA, Ramalho F, Carlotti AP, Carmona F, Alves-Filho JC, Liew FY, Cunha FQ (2019) Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit Care 23(1):113. https://doi.org/10.1186/s13054-019-2407-8
Czaikoski PG, Mota JM, Nascimento DC, Sônego F, Castanheira FV, Melo PH, Scortegagna GT, Silva RL, Barroso-Sousa R, Souto FO, Pazin-Filho A, Figueiredo F, Alves-Filho JC, Cunha FQ (2016) Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE 11(2):e0148142. https://doi.org/10.1371/journal.pone.0148142
Delano MJ, Ward PA (2016) The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev 274(1):330–353. https://doi.org/10.1111/imr.12499
Denk S, Taylor RP, Wiegner R, Cook EM, Lindorfer MA, Pfeiffer K, Paschke S, Eiseler T, Weiss M, Barth E, Lambris JD, Kalbitz M, Martin T, Barth H, Messerer DAC, Gebhard F, Huber-Lang MS (2017) Complement C5a-induced changes in neutrophil morphology during inflammation. Scand J Immunol 86(3):143–155. https://doi.org/10.1111/sji.12580
Ding Z, Du F, Averitt VR, Jakobsson G, Rönnow CF, Rahman M, Schiopu A, Thorlacius H (2021) Targeting S100A9 reduces neutrophil recruitment, inflammation and lung damage in abdominal sepsis. Int J Mol Sci 22:23. https://doi.org/10.3390/ijms222312923
Du X, Zhang M, Zhou H, Wang W, Zhang C, Zhang L, Qu Y, Li W, Liu X, Zhao M, Tu K, Li YQ (2022) Decoy nanozymes enable multitarget blockade of proinflammatory cascades for the treatment of multi-drug-resistant bacterial sepsis. Research. https://doi.org/10.34133/2022/9767643
Hawez A, Taha D, Algaber A, Madhi R, Rahman M, Thorlacius H (2022) MiR-155 regulates neutrophil extracellular trap formation and lung injury in abdominal sepsis. J Leukoc Biol 111(2):391–400. https://doi.org/10.1002/jlb.3a1220-789rr
Herrmann JB, Muenstermann M, Strobel L, Schubert-Unkmeir A, Woodruff TM, Gray-Owen SD, Klos A, Johswich KO (2018) Complement C5a receptor 1 exacerbates the pathophysiology of N meningitidis sepsis and is a potential target for disease treatment. mBio. https://doi.org/10.1128/mBio.01755-17
Janicova A, Relja B (2021) Neutrophil phenotypes and functions in trauma and trauma-related sepsis. Shock 56(1):16–29. https://doi.org/10.1097/shk.0000000000001695
Kalbitz M, Fattahi F, Herron TJ, Grailer JJ, Jajou L, Lu H, Huber-Lang M, Zetoune FS, Sarma JV, Day SM, Russell MW, Jalife J, Ward PA (2016) Complement destabilizes cardiomyocyte function in vivo after polymicrobial sepsis and in vitro. J Immunol 197(6):2353–2361. https://doi.org/10.4049/jimmunol.1600091
Kovach MA, Standiford TJ (2012) The function of neutrophils in sepsis. Curr Opin Infect Dis 25(3):321–327. https://doi.org/10.1097/QCO.0b013e3283528c9b
Liao YL, Zhou XY, Ji MH, Qiu LC, Chen XH, Gong CS, Lin Y, Guo YH, Yang JJ (2021) S100A9 Upregulation contributes to learning and memory impairments by promoting microglia M1 polarization in sepsis survivor mice. Inflammation 44(1):307–320. https://doi.org/10.1007/s10753-020-01334-6
Liu S, Wang X, She F, Zhang W, Liu H, Zhao X (2021) Effects of neutrophil-to-lymphocyte ratio combined with interleukin-6 in predicting 28-day mortality in patients with sepsis. Front Immunol 12:639735
Mai SH, Khan M, Dwivedi DJ, Ross CA, Zhou J, Gould TJ, Gross PL, Weitz JI, Fox-Robichaud AE, Liaw PC (2015) Delayed but not early treatment with DNase reduces organ damage and improves outcome in a murine model of sepsis. Shock 44(2):166–172. https://doi.org/10.1097/shk.0000000000000396
Mastellos DC, Pires da Silva BGP, Fonseca BAL, Fonseca NP, Auxiliadora-Martins M, Mastaglio S, Ruggeri A, Sironi M, Radermacher P, Chrysanthopoulou A, Skendros P, Ritis K, Manfra I, Iacobelli S, Huber-Lang M, Nilsson B, Yancopoulou D, Connolly ES, Garlanda C, Ciceri F, Risitano AM, Calado RT, Lambris JD (2020) Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin Immunol 220:108598. https://doi.org/10.1016/j.clim.2020.108598
Middleton EA, Rowley JW, Campbell RA, Grissom CK, Brown SM, Beesley SJ, Schwertz H, Kosaka Y, Manne BK, Krauel K, Tolley ND, Eustes AS, Guo L, Paine R 3, Harris ES, Zimmerman GA, Weyrich AS, Rondina MT (2019) Sepsis alters the transcriptional and translational landscape of human and murine platelets. Blood 134(12):911–923. https://doi.org/10.1182/blood.2019000067
Monneret G, Finck ME, Venet F, Debard AL, Bohé J, Bienvenu J, Lepape A (2004) The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett 95(2):193–198. https://doi.org/10.1016/j.imlet.2004.07.009
Muenstermann M, Strobel L, Klos A, Wetsel RA, Woodruff TM, Köhl J, Johswich KO (2019) Distinct roles of the anaphylatoxin receptors C3aR, C5aR1 and C5aR2 in experimental meningococcal infections. Virulence 10(1):677–694. https://doi.org/10.1080/21505594.2019.1640035
Ortiz-Espinosa S, Morales X, Senent Y, Alignani D, Tavira B, Macaya I, Ruiz B, Moreno H, Remírez A, Sainz C, Rodriguez-Pena A, Oyarbide A, Ariz M, Andueza MP, Valencia K, Teijeira A, Hoehlig K, Vater A, Rolfe B, Woodruff TM, Lopez-Picazo JM, Vicent S, Kochan G, Escors D, Gil-Bazo I, Perez-Gracia JL, Montuenga LM, Lambris JD, Ortiz de Solorzano C, Lecanda F, Ajona D, Pio R (2022) Complement C5a induces the formation of neutrophil extracellular traps by myeloid-derived suppressor cells to promote metastasis. Cancer Lett 529:70–84. https://doi.org/10.1016/j.canlet.2021.12.027
Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M, Teoh L, Van Meter L, Daum L, Lemeshow S, Hicklin G, Doig C (2004) Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med 32(11):2173–2182. https://doi.org/10.1097/01.ccm.0000145229.59014.6c
Platt CD, Zaman F, Bainter W, Stafstrom K, Almutairi A, Reigle M, Weeks S, Geha RS, Chou J (2021) Efficacy and economics of targeted panel versus whole-exome sequencing in 878 patients with suspected primary immunodeficiency. J Allergy Clin Immunol 147(2):723–726. https://doi.org/10.1016/j.jaci.2020.08.022
Prokop JW, May T, Strong K, Bilinovich SM, Bupp C, Rajasekaran S, Worthey EA, Lazar J (2018) Genome sequencing in the clinic: the past, present, and future of genomic medicine. Physiol Genomics 50(8):563–579. https://doi.org/10.1152/physiolgenomics.00046.2018
Riazuddin S, Hussain M, Razzaq A, Iqbal Z, Shahzad M, Polla DL, Song Y, van Beusekom E, Khan AA, Tomas-Roca L, Rashid M, Zahoor MY, Wissink-Lindhout WM, Basra MAR, Ansar M, Agha Z, van Heeswijk K, Rasheed F, Van de Vorst M, Veltman JA, Gilissen C, Akram J, Kleefstra T, Assir MZ, Grozeva D, Carss K, Raymond FL, O’Connor TD, Riazuddin SA, Khan SN, Ahmed ZM, de Brouwer APM, van Bokhoven H, Riazuddin S (2017) Exome sequencing of Pakistani consanguineous families identifies 30 novel candidate genes for recessive intellectual disability. Mol Psychiatry 22(11):1604–1614. https://doi.org/10.1038/mp.2016.109
Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA (2003) Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol 170(1):503–507. https://doi.org/10.4049/jimmunol.170.1.503
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet 395(10219):200–211. https://doi.org/10.1016/s0140-6736(19)32989-7
Saeidian AH, Youssefian L, Vahidnezhad H, Uitto J (2020) Research techniques made simple: whole-transcriptome sequencing by RNA-Seq for diagnosis of monogenic disorders. J Invest Dermatol 140(6):1117–1126. https://doi.org/10.1016/j.jid.2020.02.032
Shi Y, Jin Y, Li X, Chen C, Zhang Z, Liu X, Deng Y, Fan X, Wang C (2021) C5aR1 mediates the progression of inflammatory responses in the brain of rats in the early stage after ischemia and reperfusion. ACS Chem Neurosci 12(21):3994–4006. https://doi.org/10.1021/acschemneuro.1c00244
Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou E, Tsironidou V, Tsigalou C, Tektonidou M, Konstantinidis T, Papagoras C, Mitroulis I, Germanidis G, Lambris JD, Ritis K (2020) Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest 130(11):6151–6157. https://doi.org/10.1172/jci141374
Sun M, Li J, Mao L, Wu J, Deng Z, He M, An S, Zeng Z, Huang Q, Chen Z (2021) p53 Deacetylation alleviates sepsis-induced acute kidney injury by promoting autophagy. Front Immunol 12:685523. https://doi.org/10.3389/fimmu.2021.685523
Sung PS, Huang TF, Hsieh SL (2019) Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat Commun 10(1):2402. https://doi.org/10.1038/s41467-019-10360-4
Tan C, Aziz M, Wang P (2021) The vitals of NETs. J Leukoc Biol 110(4):797–808. https://doi.org/10.1002/jlb.3ru0620-375r
Wang X, Qiu L, Li Z, Wang XY, Yi H (2018) Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front Immunol 9:2456. https://doi.org/10.3389/fimmu.2018.02456
Wen H, Zhang H, Wang W, Li Y (2020) Tetrahydropalmatine protects against acute lung injury induced by limb ischemia/reperfusion through restoring PI3K/AKT/mTOR-mediated autophagy in rats. Pulm Pharmacol Ther 64:101947. https://doi.org/10.1016/j.pupt.2020.101947
Wilson AS, Randall KL, Pettitt JA, Ellyard JI, Blumenthal A, Enders A, Quah BJ, Bopp T, Parish CR, Brüstle A (2022) Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat Commun 13(1):528. https://doi.org/10.1038/s41467-022-28172-4
Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, Wei Z, Wang L, Kong L, Sun H, Ping Q, Mo R, Zhang C (2017) Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol 12(7):692–700. https://doi.org/10.1038/nnano.2017.54
Yang R, Yang H, Wei J, Li W, Yue F, Song Y, He X, Hu K (2021) Mechanisms underlying the effects of Lianhua Qingwen on sepsis-induced acute lung injury: a network pharmacology approach. Front Pharmacol 12:717652. https://doi.org/10.3389/fphar.2021.717652
Zetoune FS, Ward PA (2020) Role of complement and histones in sepsis. Front Med (Lausanne). https://doi.org/10.3389/fmed.2020.616957
Zhang W, Ye L, Wang F, Zheng J, Tian X, Chen Y, Ding G, Yang Z (2019) Immunomodulatory effects of the meretrix meretrix oligopeptide (QLNWD) on immune-deficient mice. Molecules 24:24. https://doi.org/10.3390/molecules24244452
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shen, B., Shen, Q., Zeng, Q. et al. Silenced-C5ar1 improved multiple organ injury in sepsis rats via inhibiting neutrophil extracellular trap. J Mol Histol 55, 69–81 (2024). https://doi.org/10.1007/s10735-023-10172-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-023-10172-3