Abstract
Background
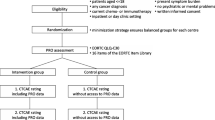
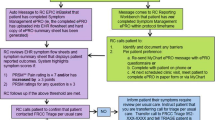
Electronic patient-reported outcomes monitoring (ePROM) is a useful communication tool for patients and healthcare providers in cancer chemotherapy. In this study, we examined the feasibility of our newly developed ePROM system, which we refer to as “Hibilog”.
Methods
An ePROM app was developed by extracting 18 items from the Patient-Reported Outcome-Common Terminology Criteria for Adverse Events (PRO-CTCAE). Symptom monitoring was conducted every two weeks for patients with metastatic breast cancer undergoing chemotherapy. The primary outcome was the response rate to the ePROM system. The secondary outcomes were response time, item missing rate, and distribution of responses for each symptom.
Results
A total of 71 cases (mean age 52.6 years) were analyzed. Performance status was 0 in 76% of the cases and 1 or higher in 24%. First-line treatment was being administered in 30% of cases, second-line treatment in 17%, and third-line or higher treatment in 53%. The response rate to the ePROM system from registration to week 40 remained high at around 80%, indicating good compliance. The average response time was 5.5 min and the missing rate for each item was below 0.4%. Among 1,093 responses, the top 3 symptoms causing interference with daily life were Fatigue (63%), Numbness and tingling (48%), and General pain (46%).
Conclusion
Our developed ePROM system was able to capture symptoms accurately in patients with metastatic breast cancer undergoing chemotherapy while maintaining a high response compliance.



Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31:1623–49. https://doi.org/10.1016/j.annonc.2020.09.010.
US Food and Drug Administration. Guidance for industry: Patient-reported outcome measures—use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf
European Medicines Agency Committee for Medicinal Products for Human Use. Pre-authorisation evaluation of medicines for human use: reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-health-related-quality-life-hrql-measures-evaluation_en.pdf
Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–55. https://doi.org/10.1200/JCO.2012.42.5967.
Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865–9. https://doi.org/10.1056/NEJMp0911494.
Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22:3485–90. https://doi.org/10.1200/JCO.2004.03.025.
Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, et al. Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer. 2009;17:1483–91. https://doi.org/10.1007/s00520-009-0613-7.
Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–24. https://doi.org/10.1200/JCO.2004.06.078.
Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027–34. https://doi.org/10.1001/jama.288.23.3027.
Greenhalgh J, Meadows K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J Eval Clin Pract. 1999;5:401–16. https://doi.org/10.1046/j.1365-2753.1999.00209.x.
Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin. 2012;62:337–47. https://doi.org/10.3322/caac.21150.
Lu SC, Porter I, Valderas JM, Harrison CJ, Sidey-Gibbons C. Effectiveness of routine provision of feedback from patient-reported outcome measurements for cancer care improvement: a systematic review and meta-analysis. J Patient Rep Outcomes. 2023;7:54. https://doi.org/10.1186/s41687-023-00578-8.
Suzuki Y, Iwamoto T, Uno M, Hatono M, Kajiwara Y, Takahashi Y, et al. Development and validation of a symptom illustration scale from the patient-reported outcome common terminology criteria for adverse events for patients with breast cancer. Breast Cancer. 2023;30:856–68. https://doi.org/10.1007/s12282-023-01480-3.
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–65. https://doi.org/10.1200/JCO.2015.63.0830.
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–8. https://doi.org/10.1001/jama.2017.7156.
Denis F, Lethrosne C, Pourel N, Molinier O, Pointreau Y, Domont J, et al. Randomized trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients. J Natl Cancer Inst. 2017. https://doi.org/10.1093/jnci/djx029.
Di Maio M, Basch E, Denis F, Fallowfield LJ, Ganz PA, Howell D, et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann Oncol. 2022;33:878–92. https://doi.org/10.1016/j.annonc.2022.04.007.
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014;106:dju244. https://doi.org/10.1093/jnci/dju244.
Miyaji T, Iioka Y, Kuroda Y, Yamamoto D, Iwase S, Goto Y, et al. Japanese translation and linguistic validation of the US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J Patient Rep Outcomes. 2017;1:8. https://doi.org/10.1186/s41687-017-0012-7.
Abernethy AP, Herndon JE 2nd, Wheeler JL, Day JM, Hood L, Patwardhan M, et al. Feasibility and acceptability to patients of a longitudinal system for evaluating cancer-related symptoms and quality of life: pilot study of an e/Tablet data-collection system in academic oncology. J Pain Symptom Manage. 2009;37:1027–38. https://doi.org/10.1016/j.jpainsymman.2008.07.011.
Berry DL, Trigg LJ, Lober WB, Karras BT, Galligan ML, Austin-Seymour M, et al. Computerized symptom and quality-of-life assessment for patients with cancer part I: development and pilot testing. Oncol Nurs Forum. 2004;31:E75-83. https://doi.org/10.1188/04.ONF.E75-E83.
Basch E, Artz D, Dulko D, Scher K, Sabbatini P, Hensley M, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol. 2005;23:3552–61. https://doi.org/10.1200/JCO.2005.04.275.
Basch E, Schrag D, Henson S, Jansen J, Ginos B, Stover AM, et al. Effect of electronic symptom monitoring on patient-reported outcomes among patients with metastatic cancer: a randomized clinical trial. JAMA. 2022;327:2413–22. https://doi.org/10.1001/jama.2022.9265.
Kawahara T, Iwamoto T, Takashima I, Hanazawa R, Uemura K, Uemura Y, et al. Association of change in health-related quality of life and treatment discontinuation in metastatic breast cancer: a post hoc, exploratory analysis of two randomized clinical trials. Support Care Cancer. 2022;30:8367–75. https://doi.org/10.1007/s00520-022-07283-0.
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33. https://doi.org/10.1056/NEJMoa1706450.
Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for breast cancer. N Engl J Med. 2022;386:1143–54. https://doi.org/10.1056/NEJMoa2115022.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab Deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. https://doi.org/10.1056/NEJMoa2203690.
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–21. https://doi.org/10.1056/NEJMoa1809615.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–28. https://doi.org/10.1016/S0140-6736(20)32531-9.
Narui K, Ishikawa T, Taira N, Uemura Y, Mukai H. Prospective cohort study of palbociclib treatment in postmenopausal patients with unresectable and metastatic hormone receptor-positive breast cancer: study protocol for a CSPOR-BC palbociclib cohort trial. World J Oncol. 2022;13:190–4. https://doi.org/10.14740/wjon1507.
Acknowledgements
We are grateful to Mr. Ohshita and Mr. Inagaki for their important contributions to development of the app.
Funding
This study was supported by the Japanese Breast Cancer Society: "Exploring the Utilization of Appropriate Patient Reported Outcomes in Breast Cancer Practice."
Author information
Authors and Affiliations
Contributions
NT and YK contributed to the study design. NT, TI, YM, KH, SY, HH, HT, DT, SK, MI, HD and TS contributed to material preparation, data collection, and analysis. NT wrote the first draft of the manuscript. All authors commented on versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest regarding the implementation of this research.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Okayama University Hospital (Date 2019.8.30/No. 1907-033).
Consent to participate
Written informed consent was obtained from all participants in the study.
Consent to publish
The authors affirm that all patients provided informed consent for publication of all figures and tables.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Taira, N., Kikawa, Y., Iwamoto, T. et al. Pilot trial of an electronic patient-reported outcome monitoring system in patients with metastatic breast cancer undergoing chemotherapy. Breast Cancer 31, 283–294 (2024). https://doi.org/10.1007/s12282-023-01537-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01537-3