Abstract
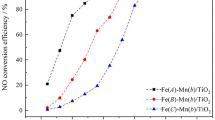
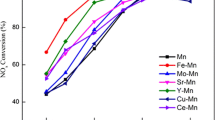
Mn/TiO2 and Fe–Mn/TiO2 catalysts were prepared using the impregnation method to explore the effect of iron doping at low temperature on the catalytic oxidation of high concentration NO by manganese-based catalysts and the catalytic reaction mechanism. This study reveals that the Fe–Mn/TiO2 catalyst performed exceptionally well in catalyzing high concentration NO smelting flue gas within a temperature range of 30–170 °C. At 150 °C, the denitrification efficiency of Fe–Mn/TiO2 (0.3) catalyst was 97.7%. Characterization analysis revealed that the incorporation of iron increased the ratio of high valence manganese and surface chemisorbed oxygen, which promoted the dispersion of surface-active substances, ultimately leading to an increase in the specific surface area of the catalyst. These factors facilitated the adsorption and activation of NH3 as well as the oxidation of NO to NO2, thus increasing the low-temperature redox capacity of the catalyst. Meanwhile, the ammonia selective catalytic reduction (NH3-SCR) denitrification mechanism over Fe–Mn/TiO2 catalysts was consistent with the Eley–Rideal (E–R) and Langmuir–Hinshelwood (L–H) mechanisms under low-temperature reaction conditions.
Graphical Abstract













Similar content being viewed by others
References
Li LH, Liu J, Cao YP (2015) Discussions on denitration technology for exhaust gas of coke oven battery. Fuel Chem Process. https://doi.org/10.16044/j.cnki.rlyhg.2015.03.016
Padmanabha RE, Neeraja E, Serhey M, Punit B, Panagiotis GS (2007) Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3. Appl Catal B: Environ 76(1–2):123–134. https://doi.org/10.1016/j.apcatb.2007.05.010
Busca G, Liettii L, Ramis G et al (1998) Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: a review. Appl Catal B: Environ 18(1–2):1–36. https://doi.org/10.1016/s0926-3373(98)00040-x
Yang SJ, Liao Y et al (2014) Competition of selective catalytic reduction and non-selective catalytic reduction over MnOx/TiO2 for NO removal: the relationship between gaseous NO concentration and N2O selectivity. Catal Sci Technol 4:224–232. https://doi.org/10.1039/c3cy00648d
Kim YJ, Kwon HJ, Nam IS et al (2010) High deNOx performance of Mn/TiO2 catalyst by NH3. Catal Today 151(3–4):244–250. https://doi.org/10.1016/j.cattod.2010.02.074
Sang ML et al (2012) Effect of the Mn oxidation state and lattice oxygen in Mn-based TiO2 catalysts on the low-temperature selective catalytic reduction of NO by NH3. J Air Waste Manag Assoc 62(9):1085–1092. https://doi.org/10.1080/10962247.2012.696532
Zfa B, Jws A, Cn A et al (2020) The insight into the role of Al2O3 in promoting the SO2 tolerance of MnOx for low-temperature selective catalytic reduction of NOx with NH3. Chem Eng J. https://doi.org/10.1016/j.cej.2020.125572
Singoredjo L, Korver R, Kapteijn F et al (2010) Alumina supported manganese oxides for the low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl Catal B Environ 1(4):297–316. https://doi.org/10.1016/0926-3373(92)80055-5
Chao HP (2019) Template-free synthesis of mesoporous Mn3O4-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. J Taiwan Inst Chem Eng. https://doi.org/10.1016/j.jtice.2018.12.006
Zhang T, Wang D, Gao Z et al (2016) Performance optimization of a MnO2/carbon nanotube substrate for efficient catalytic oxidation of low-concentration NO at room temperature. RSC Adv 6(74):70261–70270. https://doi.org/10.1039/c6RA15192B
Fang C, Zhang D, Cai S et al (2013) Low-temperature selective catalytic reduction of NO with NH3 over nanoflaky MnOx on carbon nanotubes in situ prepared via a chemical bath deposition route. Nanoscale 5(19):9199–9207. https://doi.org/10.1039/c3nr02631k
Fu MX, Li SF et al (2014) A review on selective catalytic reduction of NOx by supported catalysts at 100–300 °C-catalysts, mechanism, kinetics. Catal Sci Technol Camb 4(12):14–25. https://doi.org/10.1039/c3cy00414g
Guo N (2013) Preparation and denitrification performance of Composite Manganese catalyst [D]. Xi 'an University of Architecture and Technology
Lilian V, Christodoulos T, Antonis AZ (2011) A novel highly selective and stable Ag/MgO-CaO2-Al2O3 catalyst for the low-temperature ethanol-SCR of NO. Appl Catal B: Environ 107(1–2):164–176. https://doi.org/10.1016/j.apcatb.2011.07.010
Liu N, He F, Xie JL et al (2017) Catalytic performance of Fe-doped Mn/TiO2 catalysts for low-temperature denitration. J Synt Cryst 46(3):490–494. https://doi.org/10.16553/j.cnki.issn1000-985x.2017.03.017
Sun X, Guo RT, Liu J, Fu ZG, Liu SW, Pan WG, Shi X, Qin H, Wang ZY, Liu XY (2018) The enhanced SCR performance of Mn/TiO2 catalyst by Mo modification: Identification of the promotion mechanism. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2018.07.057
Li W, Guo RT, Wang SX, Pan WG, Chen QL, Li MY, Sun P, Liu SM (2016) The enhanced Zn resistance of Mn/TiO2 catalyst for NH3-SCR reaction by the modification with Nb. Fuel Process Technol. https://doi.org/10.1016/j.fuproc.2016.08.038
Siva SRP, Leonhard S, Anker DJ, Bernard S, Franck T, Rasmus F (2015) Mn/TiO2 and Mn–Fe/TiO2 catalysts synthesized by deposition precipitation—promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl Catal B: Environ. https://doi.org/10.1016/j.apcatb.2014.10.060
Xie HD, Chen C, He PW, Mu G, Wang KK, Yang C, Chai SN, Wang N, Ge CM (2022) Promoting H2O/SO2 resistance of Ce-Mn/TiO2 nanostructures by Sb5+/Sb3+ addition for Selective catalytic reduction of NO with NH3. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2022.154146
Liu LJ, Xu K, Su S, He LM, Qing MX, Chi HY, Liu T, Hu S, Wang Y, Xiang J (2020) Efficient Sm modified Mn/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperature. Appl Catal A: Gen. https://doi.org/10.1016/j.apcata.2020.117413
Song KL, Guo KY, Lv YX, Ma DD, Cheng YH, Shi JW (2023) Rational regulation of reducibility and acid site on Mn–Fe–BTC to achieve high low-temperature catalytic denitration performance. ACS Appl Mater Interfaces 15(3):4132–4143. https://doi.org/10.1021/acsami.2c20545
Shen B, Wang F, Liu T (2014) Homogeneous MnOx-CeO2 pellets prepared by a one-step hydrolysis process for low-temperature NH3-SCR. Power Technol 253(2):152–157. https://doi.org/10.1016/j.powtec.2013.11.015
Qiu L, Pang DD et al (2015) In situ IR studies of Co and Ce doped Mn/TiO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. Appl Surf Sci A J Devot Prop Interfaces Relat Synt BehavMater 357:189–196. https://doi.org/10.1016/j.apsusc.2015.08.259
Forzatti P (2000) Environmental catalysis for stationary applications. Catal Today. https://doi.org/10.1016/S0920-5861(00)00408-9
Jiang BQ, Lin BL, Li ZG et al (2020) Mn/TiO2 catalysts prepared by ultrasonic spray pyrolysis method for NOx removal in low-temperature SCR reaction- ScienceDirect. Colloids Surf A Physicochem Eng Aspects. https://doi.org/10.1016/j.colsurfa.2019.124210
Chen ZH, Wang FR, Li H et al (2011) Low-temperature selective catalytic reduction of NOx with NH3 over Fe-Mn mixed-oxide catalysts containing Fe3Mn3O8 phase. Ind Eng Chem Res 51(1):202–212. https://doi.org/10.1021/IE201894C
Reddy BM, Rao KN, Reddy GK et al (2006) Characterization and catalytic activity of V2O5/Al2O3-TiO2 for selective oxidation of 4-methylanisole. J Mol Catal A Chem 253(1–2):44–51. https://doi.org/10.1016/J.molcata.2006.03.016
Lohmann W (2018) Fabrication of γ-MnO2-Ce pillared montmorillonite for low temperature NH3-SCR. Z Phys Chem 232(12):1755–1769. https://doi.org/10.1515/zpch-2017-1064
Huang X, Xie A, Wu J et al (2018) Cerium modified MnTiOx/attapulgite catalyst for low-temperature selective catalytic reduction of NOx with NH3. J Mater Res 33(21):1–11. https://doi.org/10.1557/jmr.2018.242
Tang XL, Wang CZ, Gao FY, Ma YL, Yi HH, Zhao SZ, Zhou YS (2020) Effect of hierarchical element doping on the low-temperature activity of manganese-based catalysts for NH3-SCR. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2020.104399
Wang Y, Zhao R, Sun JW, Zhang K, Liu ZX, Zhao ZW, Wu WF (2022) Mechanistic study of Ce–La–Fe/γ-Al2O3 catalyst for selective catalytic reduction of NO with NH3. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2021.12.170
Pena D, Uphade B, Reddy E (2004) Smirniotis, Identification of surfacespeciesonti-tania-supported manganese, chromium, and copper oxide lowtemperature SCR catalysts. Phys Chem B 108:9927–9936. https://doi.org/10.1021/jp0313122
Chen T, Guan B et al (2014) In situ DRIFTS study of the mechanism of low temperature selective catalytic reduction over manganese-iron oxides. Chin J Catal 35(3):294–301. https://doi.org/10.1016/S1872-2067(12)60730-X
Liu N, Wang JY, Wang FY, Liu J (2018) Promoting effect of tantalum and antimony additives on deNOx performance of Ce3Ta3SbOx for NH3-SCR reaction and DRIFT studies. J Rare Earths 36(6):594
Liu H, Yan Z, Mu H et al (2022) Promotional role of the TiOx nanorod arrays as a support to load MnOx for low-temperature NH3-selective catalytic reduction of NOx: comparison of two preparation strategies. Energy Fuels 2:36
Song KL, Guo KY, Mao SM, Ma DD, Lv YX, He C, Wang HK, Cheng YH, Shi JW (2023) Insight into the origin of excellent SO2 tolerance and de-NOx performance of quasi-Mn-BTC in the low-temperature catalytic reduction of nitrogen oxide. ACS Catal 13(7):5020–5032. https://doi.org/10.1021/acscatal.3c00106
Fan ZY, Shi JW, Gao C, Gao G, Wang BR, Niu CM (2017) Rationally designed porous MnOx–FeOx Nanoneedles for low-temperature selective catalytic reduction of NOx by NH3. ACS Appl Mater Interfaces 9(19):16117–16127. https://doi.org/10.1021/acsami.7b00739
Song KL, Gao C, Lu P, Ma DD, Cheng YH, Shi JW (2023) Bimetallic modification of MnFeOx nanobelts with Nb and Nd for enhanced low-temperature de-NOx performance and SO2 tolerance. Fuel. https://doi.org/10.1016/j.fuel.2022.125861
Chen L, Junhua LI, Maofa GE (2010) DRIFT study on cerium-tungsten/titania catalyst for selective catalytic reduction of NOx with NH3. Environ Sci Technol 44(24):9590–9596. https://doi.org/10.1021/es102692b
Funding
This work is supported by the key laboratory project of Shaanxi Provincial Department of Education (Z202 00151) and the natural science fund project of Shaanxi Provincial Department of Science and Technology (2022 JM-245).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Liang, L., Ma, H. et al. Study on the Effect of Fe Doping on SCR Activity and Reaction Mechanism of Mn–TiO2 Catalysts. Catal Lett (2024). https://doi.org/10.1007/s10562-023-04524-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-023-04524-7