Abstract
This study presents the preparation of SiO2/g-C3N5@NiFe2O4 nanophotocatalyst for the removal of betamethasone from aqueous solutions. The SiO2/g-C3N5@NiFe2O4 nanophotocatalyst was synthesized using the solvothermal method, and its structure and optical properties were characterized and confirmed through XRD, FESEM, EDX, DRS, BET, VSM and PL analysis. Photocatalytic removal of betamethasone was optimized using a central composite design. The band gap of pure g-C3N5, NiFe2O4, and SiO2/g-C3N5@NiFe2O4 was obtained 2.4 eV, 2.7 eV, and 1.4 eV, respectively using the Tauc plot. The F-value of 909.88 and Lack of Fit F-value of 0.41 confirm the obtained model is significant. Also, the value of R2 = 0.9988 along with R2adja = 09977 demonstrates excellent model performance. Maximum removal efficiency of betamethasone was approximately 87.15% under the following optimal conditions: nanophotocatalyst dosage of 0.005 g/50 mL, a betamethasone concentration of 20 mg/L, and an irradiation time of 40 min under visible light. This performance closely aligns with the actual value of 80.65%. In conclusion, the SiO2/g-C3N5@NiFe2O4 nanophotocatalyst demonstrates excellent photocatalytic ability for the removal of betamethasone from aqueous solutions.
Similar content being viewed by others
Introduction
Today, the presence of pharmaceutical compounds in the environment is a pressing global concern. Pharmaceutical companies are recognized as major contributors to the introduction of pharmaceutical agents into the environment1. The presence of pharmaceuticals in wastewater poses significant and lasting challenges for humans, animals, and plants. Notably, pharmaceutical compounds and their metabolic byproducts are characterized by their potent biological activity and resistance to biodegradation2. Consequently, their persistence in the environment, resulting from continuous consumption, can lead to detrimental effects such as carcinogenesis and mutagenesis. Pharmaceutical compounds are not fully metabolized by the body and are excreted, ultimately finding their way into urban sewage systems and, subsequently, into the environment3,4. In recent years, various technologies, including advanced oxidation processes, membrane filtration methods like nanofiltration and reverse osmosis, adsorption and Microwave catalysis have been assessed for the removal of these pollutants4,5,6,7,8. Advanced oxidation processes (AOPs) are environmentally friendly cost-effective and green processes that use chemical agents such as H2O2, ozone, and semiconductors9,10 in the presence of an energy source like ultraviolet or visible light radiation to remove organic pollutants11. AOPs rely on the production of electrons and holes in the conduction band (CB) and valence band (VB). These pairs are reactive species that can attack directly to pollutants directly and can even mineralize them using generated superoxide and hydroxyl radicals12.
Numerous studies have validated the potential of advanced oxidation processes for pharmaceutical compound removal. A disadvantage of the photocatalytic process is the fast recombination of photogenerated e−/h+. To overcome this, there are various strategies such as using nano-sized materials, doping of metals/non-metals, coupling of two or more semiconductors with the suitable potential positions of CB and VB, and using plasmonic systems13. While many studies have explored the removal of pharmaceutical pollutants using AOPs, the current study aims to investigate the photocatalytic process, a crucial AOP, for the removal of betamethasone (BZ) from aqueous solutions.
The modification of 2D polymer materials based on carbon nitride to tailor their optical, electronic, and chemical properties for various applications has garnered significant attention14,15. Recently, g-C3N4 has attention as a potential photocatalyst due to its low density, and high thermal and chemical stability. However, it suffers from speedy recombination of photogenerated carriers and low surface area. To overcome these disadvantages, modification of the g-C3N4 is necessary. An effective way to modify the g-C3N4 is by changing of C/N ratio (3:5)16. This study introduces the synthesis of a novel modified carbon nitride framework with a unique stoichiometry of 3:5 C: N, referred to as g-C3N517. Research indicates that in the g-C3N5 polymer, two s-heptazine units are linked by an azo bond. Enhanced conjugation, resulting from the overlap of azo nitrogen and an increased electron density on the heptazine nucleus due to the aromatic π network of heptazine units, leads to a shift towards the upper side of the valence band maximum and, consequently, a reduction in the band gap15,18. The relatively narrow band gap and 2D structure of g-C3N5 render it an intriguing air-stable and temperature-resistant semiconductor for optoelectronic applications. Its electron-rich properties and in-plane hole character make it an effective supramolecular adsorbent for environmental applications19. g-C3N5contains one triazole and two triazine moieties, with the triazole moieties providing additional electrons and pyrrolic N sites, thereby offering more basic sites and enhancing catalytic activity20. To date, g-C3N5 based materials have found widespread use in the energy and environmental fields as a unique form of carbon nitride sample21. Nevertheless, they encounter challenges such as separation and recycling difficulties. Various techniques have been developed to modify g-C3N5, including elemental doping, defect engineering, and heterogeneity engineering20, as well as supporting g-C3N5on materials like silica, glass, zeolite, and glass fiber22. Magnetic nanoparticles have been explored as supports to facilitate the recovery and separation of photocatalysts, although they tend to reduce the photocatalytic activity of g-C3N5 due to the immobility of the photocatalyst and the low mass transfer rate. To address these issues, the use of magnetic photocatalysts comprising different molar ratios of magnetic nanoparticles and g-C3N5, along with the incorporation of an inert layer such as silica between g-C3N5and magnetic nanoparticles, allows for easy separation while preserving the photocatalytic properties of g-C3N523.
In general, numerous studies have explored the removal of pharmaceutical pollutants through advanced oxidation processes. This study, however, focuses on the synthesis of a novel photocatalyst based on g-C3N5, denoted as SiO2/g-C3N5@NiFe2O4, (SCN) and aims to assess its effectiveness in removing betamethasone (BZ) from aqueous solutions. To achieve this, the study investigates the influence of various factors, including irradiation time, nano photocatalyst dosage, initial BZ concentration, and different irradiation sources, on the removal of BZ. Design of experts (DOE) is preferred over the one-factor-at-a-time (OFAT) design of experiments, where multiple factors can be varied simultaneously, requires fewer experiments, provides more accurate estimates, and also provides the possibility to evaluate the interaction between variables24.
Results
Characterization
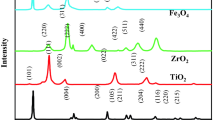
The crystal structures and crystallinity of SiO2, g-C3N5, NiFe2O4, and SiO2/g-C3N5@NiFe2O4 were assessed via XRD analysis, and the results are presented in Fig. 1. The XRD pattern of g-C3N5 displayed two distinct diffraction peaks at 2Ө = 13.1 and 27.52°, corresponding to the (100) and (002) crystal planes, which are associated with the crystalline phase of interlayer stacking and the aromatic lattice25. Additionally, the presence of a broad diffraction peak at 2Ө = 23° confirmed the amorphous nature of SiO226. The XRD spectrum of the pure NiFe2O4 sample exhibited characteristic peaks at 2Ө = 18.35, 30.2, 35.57, 37.23, 43.27, 53.71, 57.22, 62.84, and 74.36°, corresponding to the reflection planes of (111), (220), (311), (222), (400), (422), (511), (440), and (533), respectively, as per the JCPDS no. 10-032527. In the XRD spectrum of SiO2/g-C3N5@NiFe2O4, all peaks associated with the three individual samples are discernible, albeit with reduced intensity. This observation confirms the successful synthesis of the nanophotocatalyst.
The morphologies of SiO2, g-C3N5, NiFe2O4, and the SiO2/g-C3N5@NiFe2O4 nanophotocatalyst were examined using FESEM (Fig. 2). The FESEM analysis revealed that the synthesized g-C3N5 exhibits a two-dimensional nanosheet structure with a solid rock-like appearance19. The FESEM image of SiO2 displayed spherical nanospheres with uniform particle sizes of approximately 100 nm. In the case of NiFe2O4, the FESEM results showed the presence of very small particles with an average size of 40 nm, arranged in polygonal shapes due to their magnetic properties and the weak surface interaction that causes the primary particles to cluster together. This morphology provides a large surface area, where spherical SiO2 nanoparticles and polyhedral NiFe2O4 are evenly distributed in an organized and homogeneous manner, with nearly identical particle sizes, onto the g-C3N5 substrate. EDX analysis of SiO2/g-C3N5@NiFe2O4 (Fig. 2) provided qualitative composition information, confirming the successful synthesis of SiO2/g-C3N5@NiFe2O4. The elements detected in the composition include S (42.6%), Ni (20%), C (30%), N (20%), O (4%), and Fe (5%). Additionally, the TEM image of SiO2/g-C3N5@NiFe2O4 corroborates the uniform and homogeneous morphology of the synthesized SiO2/g-C3N5@NiFe2O4 nanophotocatalyst (Fig. 2).
Brunauer-Emmett-Teller (BET) analysis was applied to measure the specific surface area and pore size distribution28 of the SiO2/g-C3N5@NiFe2O4 nanophotocatalyst (Supplementary Fig. 1). As a results, the specific surface area and pore volume of the SiO2/g-C3N5@NiFe2O4 were 381.90 m2 g−1 and 4.53 cm3 g−1, respectively. It can be concluded the obtained curve belongs to the IUPAC type-IV classification29, and has a mesoporous nature. Supplementary Fig. 2 illustrates the VSM of SiO2/g-C3N5@NiFe2O4 nanophotocatalyst. The samples have magnetization in the applied field. Also, the results show that the nanophotocatalyst responds to the external magnetic field.
Optical properties
The UV-vis spectra of g-C3N5, SiO2, NiFe2O4, and SiO2/g-C3N5@NiFe2O4 were examined to assess their light absorption properties (Fig. 3a). g-C3N5 exhibited a pronounced UV optical absorption edge at 460 nm, corresponding to a band gap of 2.7 eV22. The optical absorption of NiFe2O4 at wavelengths beyond 500 nm led to a red shift in the visible light region when NiFe2O4 was modified with g-C3N5 to create a heterogeneous junction between NiFe2O4 and g-C3N5. This shift is favorable for efficient absorption under visible light, indicating that g-C3N5 can enhance the absorption properties of NiFe2O4 as a photocatalyst29. The incorporation of NiFe2O4 can increase the surface electric charge of the g-C3N5-based nanophotocatalyst, making NiFe2O4/g-C3N5 suitable as a photocatalyst under visible light conditions. Furthermore, the heterogeneous SiO2/g-C3N5@NiFe2O4 nanophotocatalyst can mitigate contact barriers and enhance electronic coupling between different semiconductors with matching energy levels, facilitating activation under visible light30,31.
The bandgap values of the synthesized samples were obtained using the Tauc model (Eq. 1)32. There are different formulas of Tauc model depending on the type of electronic transition, indirect allowed (IA), direct allowed (DA), indirect forbidden (IF) and direct forbidden (DF) transitions33.
Where h is Planck’s constant, ν is the vibrational frequency, α is the absorption coefficient, Eg is the band gap energy (eV) of the semiconductor, and A is a proportionality constant. From the Tauc diagram (Fig. 3b), the band gap of pure g-C3N5, NiFe2O4, and SiO2/g-C3N5@NiFe2O4 was calculated as 2.4 eV, 2.7 eV, and 1.4 eV, respectively. Compared with bare NiFe2O4, the bandgap of SiO2/g-C3N5@NiFe2O4 was effectively reduced, indicating that the heterojunction between NiFe2O4 and g-C3N5 can greatly improve the visible light reactivity of pure NiFe2O4. In addition, the valence band (VB) and conduction band (CB) potentials of the nanophotocatalyst were calculated using Eqs. (2) and (3), respectively30.
where, ECB and EVB are the CB and VB potentials of direct bandgap semiconductors, respectively. E° is the energy of free electrons with the hydrogen scale (~−4.5 eV vs. NHE). Therefore, Ebg is the band gap of the semiconductor (eV) calculated from the Tauc plot, and χ is the absolute electronegativity34,35.
Experimental design
Four independent variables were chosen, each with five different levels, to optimize the removal efficiency of BZ using CCD-RSM (Design-Expert® Version 7 Software DX7). The experimental design and its results are presented in Table 1. Based on these results, we established an empirical relationship between the response variable (BZ %removal) and the independent variables in the form of a quadratic polynomial equation. This equation was derived using actual values, as shown in Table 2. The ANOVA results for BZ photocatalytic removal, with a 95% confidence interval and 14 degrees of freedom, are presented in Table 3. In statistics, a model is typically considered significant when it has a large F-value and a small p-value (<0.05). In our case, the F-value of 909.88 suggests the model’s significance. Specifically, the model terms A, B, C, D, AB, AC, AD, BC, BD, CD, A2, B2, C2, and D2 all have p-values < 0.05, indicating their significance. The coefficient of determination (R²), which quantifies the proportion of response variation explained by the model, reflects the goodness of fit. An R2 value close to 1 indicates a strong agreement between predicted and experimental data. In our research, R2 values of 0.9988 along with a satisfactory R2adja = 0.997, demonstrate excellent model performance36. Additionally, the adequate precision (Adeq Precision) value of 122.091 confirms the model’s ability to predict results effectively. The F-value of 0.41 suggests that the Lack of Fit is not significant relative to pure error37,38, indicating an 88.89% chance that such a large Lack of Fit F-value could occur due to noise. To assess the model’s repeatability, we consider the coefficient of variation (CV), which compares the estimated standard error to the average observed response. A model can be considered reproducible if its CV is not more than 10%. In our case, the proposed model exhibits good reproducibility with a coefficient of variation of 1.37%.
Three-dimensional plots illustrating the influence of independent factors are depicted in Fig. 4. Notably, irradiation time stands out as a crucial parameter directly impacting removal efficiency. Increasing the irradiation time from 10 to 50 min provides photocatalyst particles with more opportunities to engage in photocatalytic reactions. At lower irradiation times, the likelihood of collisions between BZ molecules and the catalyst surface is diminished. As time increases, this probability rises, resulting in enhanced BZ removal. Furthermore, extended irradiation time leads to a higher count of excited particles, consequently yielding more radicals and positive holes. This increase in active species contributes to a higher percentage of BZ removal39. In Fig. 4a, it is evident that elevating the photocatalyst dosage from 0.001 g/50 mL to 0.005 g/50 mL results in an increase in removal efficiency approximately from 45% to 88%. This improvement can be attributed to the augmented number of active sites, which enhances the adsorption of BZ on the SNC surface. Moreover, an increased number of active sites enhances light absorption and the production of active species such as •OH, •O2, and h+. In essence, at lower dosages of nanophotocatalyst, the catalyst absorbs fewer photons for the photocatalytic reaction, leading to decreased photocatalytic activity. Conversely, increasing the dosage of photocatalyst boosts the number of active sites on the catalyst surface, resulting in increased BZ removal 9.
In Fig. 4b, it is evident that an increase in the initial BZ concentration from 10 to 50 mg/L results in a decrease in BZ removal. This observation can be attributed to the fact that as the initial concentration of BZ rises, BZ molecules tend to adsorb onto the catalyst’s surface, causing a significant amount of UV light to be absorbed by BZ molecules instead of the photocatalyst. Consequently, light penetration to the catalyst surface diminishes. Moreover, the production of hydroxyl radicals decreases since active sites become occupied by BZ. Additionally, the adsorbed BZ on the photocatalyst hinders the reaction of adsorbed molecules with photo-generated holes or hydroxyl radicals due to the lack of direct contact between the semiconductor and these species. Furthermore, as the initial BZ concentration increases, the catalyst surface area required for removal also increases. This is because irradiation time, the dosage of the photocatalyst, and the generation of •OH on the photocatalyst’s surface remain constant. Consequently, BZ removal decreases with an increase in BZ concentration30. A noteworthy aspect of this study is the investigation of different light sources’ effects on BZ removal using SCN nanophotocatalyst. The results indicate that UV-C sources and darkness have the most and least significant impacts on the photocatalytic removal of BZ, respectively. UV-C, characterized by the shortest wavelength (100-280 nm) among the three forms of UV (UVB and UVA being the others), has the most potent effect and is commonly employed, often in the form of mercury lamps, for degrading pollutants. As shown in Fig. 4b, the highest BZ removal is achieved under UV-C illumination, consistent with findings from previous research 32. Moreover, Fig. 4b demonstrates that the SCN nanophotocatalyst remains active even under visible light conditions. To investigate the influence of active species on BZ removal during the photocatalytic process, we introduced scavengers, including isopropanol (IPA), ethylenediaminetetraacetic acid disodium salt (EDTA), and benzoquinone (BQ), each at a concentration of 0.2 mmol/L. These scavengers are known as •OH, h+, and\({\,\bullet O}_{2}^{-}\), respectively 33. As depicted in Fig. 5, a significant decrease in BZ degradation efficiency was observed in the presence of IPA and EDTA. This finding confirms that both •OH and h+ play primary roles in BZ degradation 30.
Photoluminescence Spectroscopy (PL) is employed to investigate the trapping, migration, transport, and recombination rate of photogenerated electron-hole pairs in the prepared photocatalyst. Typically, a lower PL intensity signifies a reduced photocarrier recombination rate, leading to higher photocatalytic activity. As depicted in Fig. 6, both g-C3N5 and NiFe2O4 exhibit photoluminescence at 325 nm, with NiFe2O4 displaying higher intensity than g-C3N5. Notably, the introduction of g-C3N5 into NiFe2O4 further diminishes the PL spectrum’s intensity. This decrease indicates that photogenerated electron-hole pairs can be more effectively and efficiently separated. These results suggest that the SiO2/g-C3N5@NiFe2O4 nanophotocatalyst facilitates the efficient transfer of photogenerated electron-hole pairs at the interface of its heterogeneous structure under visible light irradiation. Consequently, the rate of electron-hole recombination decreases, resulting in the highest photocatalytic activity under visible light irradiation. In the PL spectrum shown in Fig. 6, the weaker peak of SiO2/g-C3N5@NiFe2O4 compared to g-C3N5 and NiFe2O4 signifies the greater separation of photogenerated electron-hole pairs, which is beneficial for photodegradation.
Proposed photocatalytic mechanism
To investigate the photodegradation mechanism of BZ using the SiO2/g-C3N5@NiFe2O4 nano photocatalyst, we calculated the conduction band (ECB) and valence band (EVB) potentials of NiFe2O4 (ECB = 0.35 eV and EVB = 2.33 eV) and g-C3N5 (ECB = −0.33 eV and EVB = +1.44 eV) using Eqs. (3) and (4). Subsequently, we proposed a mechanism for the photodegradation of BZ. As illustrated in Fig. 7, under visible light irradiation, electrons in the valence band (VB) of both g-C3N5 and NiFe2O4 are excited to the conduction band (CB). Subsequently, electrons in the CB of g-C3N5 transfer to the CB of NiFe2O4, while holes in the VB of NiFe2O4 transfer to the VB of g-C3N5. This electron and hole redistribution effectively prevents the rapid recombination of photoinduced carriers. Moreover, the CB potential of NiFe2O4 (0.35 eV) is less negative than E°(O2/•O2−) (−0.046 eV vs. NHE), indicating that O2 is not reduced by electrons to produce •O2− on the photocatalyst surface. On the other hand, the excited electrons on the CB of g-C3N5 may generate °O2− radicals and the excess electrons travel to the CB of NiFe2O4. So, the O2 molecules can react with H+ ions and electrons on CB of NiFe2O4 and produce H2O2 molecules 40,41. The produced H2O2 can react with electrons and produce •OH. Then, the generated radicals react with BZ and produce final products (Eqs. 4–7).
Additionally, compared to E°(•OH/OH−) (2.38 eV vs. NHE), the VB potential of g-C3N5 (1.44 eV) is less positive, implying that •OH cannot oxidize H2O with photogenerated holes (Eq. 8) 42. Instead, the holes directly react with adsorbed BZ on the surface of SiO2/g-C3N5@NiFe2O4.
Furthermore, since the CB potential for g-C3N5 is more negative, photogenerated electrons can quickly move from the CB of g-C3N5 to that of NiFe2O4, and the holes in the VB of NiFe2O4 are transferred to the VB of g-C3N5. This efficient charge separation effectively inhibits electron-hole recombination 42,43,44. The heterogeneous structure of SiO2/g-C3N5@NiFe2O4 contributes to the prevention of electron-hole pair recombination, thereby enhancing BZ removal under visible light. Therefore, it can be concluded that •OH and h+ are primarily responsible for increasing the photocatalytic activity of SiO2/g-C3N5@NiFe2O4 under visible light.
Assessing the stability and recyclability of the photocatalyst is crucial from an economic perspective. Therefore, we investigated the photocatalytic activity of the prepared photocatalyst after recycling. The photocatalyst was washed with deionized water and reused for the removal of BZ under visible light. The photocatalytic recyclability of SCN was evaluated over four cycles. As depicted in Fig. 8, the removal capacity of the nanophotocatalyst decreased up to 16.53% after four consecutive cycles. This indicates that the SCN nano photocatalyst exhibits exceptional photostability and maintains high degradation efficiency over multiple cycles. This characteristic is highly advantageous for catalysts in practical applications.
Optimization
Our ultimate objective is to achieve the maximum removal of BZ. Based on the central composite design, the recommended optimal conditions for BZ removal are as follows: a SCN dosage of 0.005 g/50 mL, a BZ concentration of 20 mg/L, an irradiation time of 40 minutes, and the use of visible light as the light source. Under these optimal conditions, the percentage removal of BZ reaches 87.15%, which is closely aligned with the BZ removal predicted by the model (80.65%).
Discussion
In this study, we synthesized the SiO2/g-C3N5@NiFe2O4 nano photocatalyst and utilized it for the removal of BZ from aqueous solutions through the application of central composite design. We selected four independent parameters: nano photocatalyst dosage, initial BZ concentration, irradiation time, and light source. According to the ANOVA results, the most significant factors affecting BZ removal were BZ concentration and the choice of light source. The predicted model exhibited an excellent fit with a regression coefficient (R2 = 0.9988). Under optimal conditions, we achieved an impressive 87.15% removal of BZ.
Methods
Experimental methods
Iron (III) nitrate. 9H2O, Potassium Hydroxide, Tetraethoxysilane (TEOS), Ammonium, 3-Amino-1,2,4-triazole (3-AT) and Nickel (II) nitrate. 6H2O and ethanol were purchased from Merck company. Betamethasone sodium phosphate (BZ) was obtained from the Alborz company in Iran.
Synthesis of g-C3N5
The preparation of g-C3N5 was completed by a simple thermal polymerization method. Briefly, two-dimensional metal-free g-C3N5 nanosheets were obtained by polymerization of 5 g of 3-amino-1, 2, 4-triazole (3-AT) at 500 °C for 3 h in a crucible with a ramping rate of 5 °C/min24. The g-C3N5 was obtained after cooling and ground for further use.
Synthesis of SiO2
Firstly, 30 ml of tetraethoxysilane (TEOS) and distilled water were mixed and stirred for 30 min at room temperature. 300 mL of ethanol and 15 mL of NH3 were added immediately to the solution under stirring for 24 h. The obtained solution was filtered, washed three times with water/ ethanol, and dried at 60 °C.
Synthesis of SiO2/g-C3N5@NiFe2O4
The SiO2/g-C3N5@NiFe2O4 nanophotocatalyst was prepared via a simple method. Briefly, the 0.2 g of SiO2, 0.2 g of g-C3N5, and 10 ml of water were mixed and placed in an ultrasonic bath for half an hour until the materials were completely dispersed. Then, 1.62 g of Fe(NO3)2·9H2O, 0.58 g of Ni(NO3)2·6H2O, and 15 mL of distilled water were added and stirred for one hour at room temperature. 25 ml of KOH (2 M) was added drop by drop to the above solution while stirring and continuously vigorously for 1 h. The obtained solution was poured into a Teflon-lined stainless steel autoclave and placed in the oven at 160 °C for 12 h. The resulting product was filtered and washed 3 times with distilled water/ethanol and then dried at 50 °C.
Experimental method
The experiments were conducted within a semi-batch system. Four independent variables, namely photocatalyst dosage, radiation sources, irradiation time, and BZ concentration were investigated at five different levels. The experimental design was based on the central composite design, utilizing Design-Expert® Version 7 software (DX7), resulting in the formulation of 30 experiments (as detailed in Table 1). The Design-Expert software was employed to analyze the combined effects of all four variables on the photocatalytic removal efficiency of BZ. For the illumination of the experiments, a halogen lamp with an output power of 300 W was employed as the visible light source. The UVC lamp utilized in the experiments had a power rating of 8 W and emitted light at a wavelength of 254 nm. The UVB radiation source was obtained from Philips, with a power rating of 20 W, Sunlamp, Holland, and the UVA bulb lamp, with a power rating of 25 W, was purchased from OSRAM. During the photocatalytic removal of BZ, the solution was mixed by a magnetic stirrer, sampling was done at specified times, and the removal percent of BZ was determined at a wavelength of 243 nm using a UV-vis spectrophotometer. The Eq. (9) was used to calculate the % of BZ removal:
Here, R(%) is the percentage of BZ removal by photocatalyst, C0 is the initial and Ct is the final concentration of BZ, respectively.
Analysis
The synthesized nanophotocatalyst was analyzed by the XRD model (X’pertpro) of Panalytical company. The morphology of nanocomposite was determined with a field emission scanning electron microscope (FESEM-EDX-Mapping), ZEISS, Germany, model sigma VP, and a scanning electron microscope (TEM) model EM10C-100KV from Zeiss, Germany. To measure the optical properties and calculate the band gap of the synthesized nanocomposite, the UV/vis-DRS model uv-2550 of Shimadzu Company, Japan was used. PL analysis was recorded using Cary Eclipse fluorescence spectrometer of Varian, America. The concentration of BZ was recorded by UV-Vis spectrophotometer (Hach model 2800 DR).
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Mehrizad, A. et al. Comparison of 4-chloro-2-nitrophenol adsorption on single-walled and multi-walled carbon nanotubes. Iran. J. Environ. Health Sci. Eng. 9, 1–6 (2012).
Shahnazari-Shahrezaie, E. & Nezamzadeh-Ejhieh, A. A zeolite modified carbon paste electrode based on copper exchanged clinoptilolite nanoparticles for voltammetric determination of metronidazole. RSC Adv. 7, 14247–14253 (2017).
Nosuhi, M. & Nezamzadeh-Ejhieh, A. Comprehensive study on the electrocatalytic effect of copper–doped nano-clinoptilolite towards amoxicillin at the modified carbon paste electrode–solution interface. J. Colloid Interface Sci. 497, 66–72 (2017).
Yu, J. et al. Oxygen-deficient engineering for perovskite oxides in the application of AOPs: Regulation, detection, and reduction mechanism. Catalysts 13, 148 (2023).
Wang, Y. et al. Enhanced removal of Cr (VI) from aqueous solution by stabilized nanoscale zero valent iron and copper bimetal intercalated montmorillonite. Colloid Interface Sci. 606, 941–952 (2022).
Li, H. et al. Perovskite catalysts with different dimensionalities for environmental and energy applications: a review. Sep. Purif. Technol. 307, 122716 (2023).
Gharbani, P., Mehrizad, A. & Jafarpour, I. Adsorption of penicillin by decaffeinated tea waste. Pol. J. Chem. Technol. 17, 95–99 (2015).
Wang, Y. et al. Construction of microwave/PMS combined dual responsive perovskite-MXene system for antibiotic degradation: Synergistic effects of thermal and non-thermal. Appl. Surf. Sci. 639, 158263 (2023).
Yin, W. et al. Synthesis of carbon microsphere-supported nano-zero-valent iron sulfide for enhanced removal of Cr (VI) and p-nitrophenol complex contamination in peroxymonosulfate system”. J. Mol. Liq. 390, 123089 (2023).
Derikvandi, H. & Nezamzadeh-Ejhieh, A. ncreased photocatalytic activity of NiO and ZnO in photodegradation of a model drug aqueous solution: effect of coupling, supporting, particles size and calcination temperature. J. Hazard. Mater. 321, 629–638 (2017).
Vahabirad, S., Nezamzadeh-Ejhieh, A. & Mirmohammadi, M. A co-precipitation synthesized BiOI/(BiO) 2CO3 nanocatalyst: An experimental design and mechanism study towards photodegradation of sulfasalazine. J. Taiwan Inst. Chem. Eng. 151, 105139 (2023).
Fathi, E. & Gharbani, P. Modeling and optimization removal of reactive Orange 16 dye using MgO/g-C3N4/zeolite nanocomposite in coupling with LED and ultrasound by response surface methodology. Diam. Relat. Mater. 115, 108346 (2021).
Yousefi, A. & Nezamzadeh-Ejhieh, A. Preparation and characterization of SnO2-BiVO4-CuO catalyst and kinetics of phenazopyridine photodegradation. Iran. J. Catal. 11, 247–259 (2021).
Ghattavi, S. & Nezamzadeh-Ejhieh, A. A visible light driven AgBr/g-C3N4 photocatalyst composite in methyl orange photodegradation: focus on photoluminescence, mole ratio, synthesis method of g-C3N4 and scavengers. Compos. B Eng. 183, 107712 (2020).
Ghattavi, S. & Nezamzadeh-Ejhieh, A. GC-MASS detection of methyl orange degradation intermediates by AgBr/g-C3N4: Experimental design, bandgap study, and characterization of the catalyst. Int. J. Hydrogen Energ. 45, 24636–24656 (2020).
Liu, T. et al. Preparation of C3N5 nanosheets with enhanced performance in photocatalytic methylene blue (MB) degradation and H2-evolution from water splitting. Environ. Res. 188, 109741 (2020).
Fard, B. H., Khojasteh, R. R. & Gharbani, P. Preparation and characterization of visible-light sensitive nano Ag/Ag3VO4/AgVO3 modified by graphene oxide for photodegradation of reactive orange 16 Dye. J. Inorg. Organomet. Polym. Mater. 28, 1149–1157 (2018).
Park, D. et al. Energy efficient synthesis of ordered mesoporous carbon nitrides with a high nitrogen content and enhanced CO2 capture capacity. Chem. Eur. J. 23, 10753–10757 (2017).
Cai, Z. et al. Type-II surface heterojunction of bismuth-rich Bi4O5Br2 on nitrogen-rich g-C3N5 nanosheets for efficient photocatalytic degradation of antibiotics. Sep. Purif. Technol. 280, 119772 (2022).
Mortazavi, B. et al. Nanoporous C3N4, C3N5 and C3N6 nanosheets; novel strong semiconductors with low thermal conductivities and appealing optical/electronic properties. Carbon N. Y. 167, 40–50 (2020).
Wu, L. et al. Three-dimensional C3N5/RGO aerogels with enhanced visible-light response and electron-hole separation efficiency for photocatalytic uranium reduction. Chem. Eng. J. 427, 131773 (2022).
Vadivel, S., Hariganesh, S., Paul, B., Mamba, G. & Puviarasu, P. Highly active novel CeTi2O6/g-C3N5 photocatalyst with extended spectral response towards removal of endocrine disruptor 2, 4-dichlorophenol in aqueous medium. Colloids Surf. A Physicochem. Eng. Asp. 592, 124583 (2020).
Vadivel, S. et al. Synthesis of novel AgCl loaded g-C3N5 with ultrahigh activity as visible light photocatalyst for pollutants degradation. Chem. Phys. Lett. 738, 136862 (2020).
Ghahremani, P., Nezamzadeh-Ejhieh, A. & Vakili, M. H. A comparison of adsorption capacity of several synthesis methods of cellulose-based absorbent towards Pb (II) removal: Optimization with response surface methodology. Int. J. Biol. Macromol. 253, 127115 (2023).
Liu, Y. et al. NiFe2O4/g-C3N4 heterojunction composite with enhanced visible-light photocatalytic activity. J. Saudi Chem. Soc. 22, 439–448 (2018).
Sun, J., Xu, Z., Li, W. & Shen, X. Effect of nano-SiO2 on the early hydration of alite-sulphoaluminate cement. Nanomaterials. 7, 102 (2017).
Mishra, P., Behera, A., Kandi, D. & Parida, K. Facile construction of a novel NiFe 2 O 4@ P-doped gC 3 N 4 nanocomposite with enhanced visible-light-driven photocatalytic activity. Nanoscale Adv. 1, 1864–1879 (2019).
Zhang, X. et al. Effect of benzoic acid and dopamine hydrochloride as a modulator in the water resistance of Universitetet i Oslo-67: adsorption performance and mechanism. J. Colloid Interface Sci. 651, 424–435 (2023).
AttariKhasraghi, N., Zare, K., Mehrizad, A., Modirshahla, N. & Behnajady, M. A. Achieving the enhanced photocatalytic degradation of ceftriaxone sodium using CdS-g-C3N4 nanocomposite under visible light irradiation: RSM modeling and optimization. J. Inorg. Organomet. Polym. Mater. 31, 3164–3174 (2021).
Soleimani, F. & Nezamzadeh-Ejhieh, A. Study of the photocatalytic activity of CdS–ZnS nano-composite in the photodegradation of rifampin in aqueous solution. J. Mater. Res. Technol. 9, 16237–16251 (2020).
Foroughipour, M. & Nezamzadeh-Ejhieh, A. CaTiO3/g-C3N4 heterojunction-based composite photocatalyst: Part I: Experimental design, kinetics, and scavenging agents’ effects in photocatalytic degradation of gemifloxacin. Chemosphere. 334, 139019 (2023).
Farsi, M. & Nezamzadeh-Ejhieh, A. A Z-scheme Cobalt (II) oxide-silver tungstate nano photocatalyst: experimental design and mechanism study for the degradation of methylene blue. Surf. Interfaces 32, 102148 (2022).
Mehrabanpour, N., Nezamzadeh-Ejhieh, A., Ghattavi, S. H. & Ershadi, A. A magnetically separable clinoptilolite supported CdS-PbS photocatalyst: characterization and photocatalytic activity toward cefotaxime. Appl. Surf. Sci. 614, 156252 (2023).
Ghattavi, S. & Nezamzadeh-Ejhieh, A. A double-Z-scheme ZnO/AgI/WO3 photocatalyst with high visible light activity: experimental design and mechanism pathway in the degradation of methylene blue. J. Mol. Liq. 322, 114563 (2021).
Vahabirad, S. & Nezamzadeh-Ejhieh, A. 2023. Evaluation of the photodegradation activity of bismuth oxoiodide/bismuth sub-carbonate nanocatalyst: experimental design and the mechanism study. Ecotoxicol. Environ. Saf. 263, 115254 (2023).
Eshraghi, F. & Nezamzadeh-Ejhieh, A. EDTA-functionalized clinoptilolite nanoparticles as an effective adsorbent for Pb (II) removal. Environ. Sci. Pollut. Res. 25, 14043–14056 (2018).
Tamiji, T. & Nezamzadeh-Ejhieh, A. A comprehensive study on the kinetic aspects and experimental design for the voltammetric response of a Sn (IV)-clinoptilolite carbon paste electrode towards Hg (II). J. Electroanal. Chem. 829, 95–105 (2018).
Nosuhi, M. & Nezamzadeh-Ejhieh, A. High catalytic activity of Fe (II)-clinoptilolite nanoparticales for indirect voltammetric determination of dichromate: experimental design by response surface methodology (RSM). Electrochimica Acta 223, 47–62 (2017).
Gharbani, P. “Synthesis of polyaniline-tin (II) molybdophosphate nanocomposite and application of it in the removal of dyes from aqueous solutions.”. J. Mol. Liq. 242, 229–234 (2017).
Arabpour, N. & Nezamzadeh-Ejhieh, A. Photodegradation of cotrimaxazole by clinoptilolite- supported nickel oxide. Process Saf. Environ. Prot. 102, 431–440 (2016).
Rezaei, M. & Nezamzadeh-Ejhieh, A. The ZnO-NiO nano-composite: a brief characterization, kinetic and thermodynamic study and study the Arrhenius model on the sulfasalazine photodegradation. Int. J. Hydrogen Energ. 45, 24749–24764 (2020).
Hemmatpour, P. & Nezamzadeh-Ejhieh, A. A Z-scheme CdS/BiVO4 photocatalysis towards Eriochrome black T: an experimental design and mechanism study. Chemosphere 307, 135925 (2022).
Pourtaheri, A. & Nezamzadeh-Ejhieh, A. Photocatalytic properties of incorporated NiO onto clinoptilolite nano-particles in the photodegradation process of aqueous solution of cefixime pharmaceutical capsule. Chem. Eng. Res. Des. 104, 835–843 (2015).
Mirsalari, S. A., Nezamzadeh-Ejhieh, A. & Massah, A. R. A Z-scheme CdS/Ag3PO4 catalyst: characterization, experimental design and mechanism consideration for methylene blue. Spectrochim. Acta A. 288, 122139 (2023).
Author information
Authors and Affiliations
Contributions
The paper was written with the contributions of all authors. All authors have given approval for the final version of the paper. Neda Bagherlou designed and executed the experiments. Parvin Gharbani and Ali Mehrizad performed modeling and analysis. Elnaz Ghasemi and Mirzaagha Babazadeh provided materials analysis and characterization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bagherlou, N., Ghasemi, E., Gharbani, P. et al. Optimization and modeling of betamethasone removal from aqueous solutions using a SiO2/g-C3N5@NiFe2O4 nanophotocatalyst by RSM. npj Clean Water 7, 2 (2024). https://doi.org/10.1038/s41545-023-00295-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-023-00295-1