Abstract
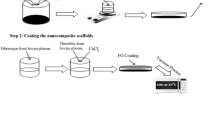
Engineered cardiac constructs (ECC) aid in the progression of regenerative medicine, disease modeling and targeted drug delivery to adjust and aim the release of remedial combination as well as decrease the side effects of drugs. In this research, polycaprolactone/gold nanoparticles (PCL/GNPs) three-dimensional (3D) composite scaffolds were manufactured by 3D printing using the fused deposition modeling (FDM) method and then coated with gelatin/spironolactone (GEL/SPL). Scanning electron microscopy (SEM) and Fourier transform-infrared spectroscopy (FTIR–ATR) were applied to characterize the samples. Furthermore, drug release, biodegradation, behavior of the myoblasts (H9C2) cell line, and cytotoxicity of the 3D scaffolds were evaluated. The microstructural observation of the scaffolds reported interconnected pores with 150–300 µm in diameter. The 3D scaffolds were degraded significantly after 28 days of immersion in stimulated body fluid (SBF), with the maximum rate of GEL- coated 3D scaffolds. SPL release from cross-linked GEL coating demonstrated the excess of drug release over time, and according to the control release systems, the drug delivery systems (DDS) went into balance after the 14th day. In addition, cell culture study showed that with the addition of GNPs, the proliferation of (H9C2) was enhanced, and with GEL/SPL coating the cell attachment and viability were improved significantly. These findings suggested that PCL/GNPs 3D scaffolds coated with GEL/SPL can be an appropriate choice for myocardial tissue engineering.








Similar content being viewed by others
Data Availability
Data will be available on request.
References
Mirotsou, M., Jayawardena, T. M., Schmeckpeper, J., Gnecchi, M., & Dzau, V. J. (2011). Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. Journal of MolEcular and Cellular Cardiology, 50(2), 280–289.
Zimmermann, W. H., Melnychenko, I., & Eschenhagen, T. (2004). Engineered heart tissue for regeneration of diseased hearts. Journal of Biomaterials Applications, 25(9), 1639–1647.
Derby, B. (2012). Printing and prototyping of tissues and scaffolds. Science, 338(6109), 921–926.
Jammalamadaka, U., & Tappa, K. (2018). Recent advances in biomaterials for 3D printing and tissue engineering. Journal of Functional Biomaterials, 9, 22.
Sigaux, N., Pourchet, L., Breton, P., Brosse, S., Louvrier, A., & Marquette, C. A. (2019). 3D bioprinting: Principles, fantasies and prospects, Journal of Stomatology. Oral and Maxillofacial Surgery, 120, 128–132.
Possl, A., Hartzke, D., Schmidts, T. M., Runkel, F. E., & Schlupp, P. (2021). A targeted rheological bioink development guideline and its systematic correlation with printing behavior. Biofabrication, 13, 1–16.
Xiao, Y., Wang, L., Lou, K., Yang, Y., Zhang, P., & Li, J. (2022). 3D biocompatible polyester blend scaffolds containing degradable calcium citrate for bone tissue engineering. Journal of Bionic Engineering, 19, 497–506.
Luca, A. C., Mersch, S., Deenen, R., Schmidt, S., Messner, I., Schafer, K. L., Baldus, S. E., Huckenbeck, W., Piekorz, R. P., Knoefel, W. T., Krieg, A., & Stoecklein, N. H. (2013). Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cell lines. PLoS One, 8(3), e59689.
Xiong, X., Chen, Y., Yuan, R., Qiu, G., Weir, M. D., Xu, H. H. K., Liu, J., Ruan, J., Chang, X., & Qu, S. (2022). 3D printed mechanically strong calcium phosphate cement scaffolds with metformin/stem cell-encapsulating alginate microbeads for bone tissue engineering. Journal of Bionic Engineering, 19, 1658–1670.
Hubbell, J. A. (1995). Biomaterials in tissue engineering. Journal of Biotechnology, 13(6), 565–576.
Santoro, M., Tatara, A. M., Mikos, A. G., & A. G. (2014). Gelatin carriers for drug and cell delivery in tissue engineering. Journal of Control Release, 190, 210–218.
Tang, Z. G., Black, R. A., Curran, J. M., Hunt, J. A., Rhodes, N. P., & Wiliams, D. F. (2004). Surface properties and biocompatibility of solvent-cast poly Ɛ-caprolactone films. Journal of Biomaterials Applications, 25(19), 4741–4748.
Lim, G. J., Zare, S., Dyke, M. V., & Atala, A. (2010). Cell microencapsulation. Advanced in Experimental Medicine and Biology, 670, 126–136.
Hasan, A., Morshed, M., Memic, A., Hassan, S., Webster, T. J., & Marei, H. (2018). Nanoparticles in tissue engineering: Applications, challenges and prospects. International Journal of Nanomedicine, 13, 5637–5655.
Shevach, M., Moaz, B., Feiner, R., Shapira, A., & Dvir, T. (2013). Nanoengineering gold particle composite fibers for cardiac tissue engineering. Journal of Materials Chemistry B, 39, 1–8.
Santos, E., Zarate, J., Orive, G., Hernandez, R. M., & Pedraz, J. L. (2010). Biomaterials in cell microencapsulation. Advanced in Experimental Medicine and Biology, 670, 5–21.
Schoener, C. A., Hutson, H. N., & Peppas, N. A. (2012). PH-responsive hydrogels with dispersed hydrophobic nanoparticles for the delivery of hydrophobic therapeutic agents. Polymer International, 61, 874–879.
Laha, A., Sharma, C. S., & Majumdar, S. (2017). Sustained drug release from multi-layered sequentially crosslinked electrospun gelatin nanofiber mesh. Materials Science and Engineering C, 76, 782–786.
Han, K. H., Kang, Y. S., Han, S. Y., Jee, Y. H., Lee, M. H., Han, J. Y., Kim, H. K., Kim, Y. S., & Cha, D. R. (2006). Spironolactone ameliorates renal injury and connective tissue growth factor expression in type II diabetic rats. International Society of Nephrology, 70, 111–120.
Hermidorff, M. M., Faria, O. G., Amancio, C. G., Assis, M., & Isoldi, M. C. (2015). Non-genomic effects of spironolactone and eplerenone in cardiomyocytes of neonatal Wistar rats: Do they evoke cardioprotective path ways? International Journal of Biochemistry & Cell Biology, 93, 83–93.
McDiarmid, A. K., Swoboda, P. P., Erhayiem, B., Bounford, K. A., Bijsterveld, P., Tyndall, K., Fent, G. J., Garg, P., Dobson, L. E., Musa, T. A., Foley, J. R. J., Witte, K. K., Kearney, M. T., Greenwood, J. P., & Plein, S. (2020). Myocardial effects of aldosterone antagonism in heart failure with preserved ejection fraction. Journal of the American Heart Association, 9, 15–21.
Azmy, B., Standen, G., Kristova, P., Flint, A., Lewis, A. L., & Salvage, J. P. (2017). Nanostructured DPA-MPC-DPA triblock copolymer gel for controlled drug release of ketoprofen and spironolactone. Journal of PharmAcy and Pharmacology, 69, 978–990.
Ghaziof, S. H., Shojaei, S. H., Mehdikhani, M., Khodaei, M., & Nodoushan, M. J. (2022). Electro-conductive 3D printed polycaprolactone/gold nanoparticles nanocomposite scaffolds for myocardial tissue engineering. Journal of Mechanical Behavior of Biomedical Materials, 132, 105–271.
Khodaei, M., Amini, K., & Valanezhad, A. (2020). Fabrication and characterization of poly lactic asid scaffolds by fused deposition modeling for bone tissue engineering. Journal Wuhan University of Technology, Materials Science Edition, 35, 248–251.
Jensen, A., Lim, L. T., Barbut, S., & Marcone, M. F. (2015). Development and characterization of soy protein films incorporated with cellulose fibers using a hot surface casting technique. LWT-Food Science andTechnology, 60, 162–170.
Mehdikhani, M., & Ghaziof, S. H. (2018). Electrically conductive poly-Ɛ-caprolactone/polyethylene glycol/multi wall carbon nanotube nanocomposite scaffolds coated with fibrin glue for myocardial tissue engineering. Journal of Applied Physics, 124(77), 1–15.
Meerloo, J., Kaspers, G. J., & Cloos, J. (2011). Cell sensivity assays: The MTT assay. Method, Molecular Biology, 731, 237–245.
Arastouei, M., Khodaei, M., Atyabi, S. M., & Nodoushan, M. J. (2021). The in-vitro biological properties of 3D poly lactic acid/akermanite composite porous scaffold for bone tissue engineering. Materials Today Communications, 27, 102–176.
Son, S. R., Franco, A. A., Bae, S. H., Min, Y. K., & Lee, B. T. (2013). Electrospun PLGA/gelatin fibrous tubes for the application of biodegradable intestinal stent in rat model. Journal of Biomedical Materials Research Part B Applied Biomaterials, 101, 1095–1105.
Gorodzha, S. N., Surmeneva, M. A., & Surmenev, R. A. (2015). Fabrication and characterization of polycaprolactone cross- linked and highly-aligned 3D artificial scaffolds for bone tissue regeneration via electrospinning technology. Materials Science and Engineering, 98, 1–7.
Resende, R. C., Viana, O., Freitas, J. T. J., Bonfilio, R., Ruela, A. L. M., & Araujo, M. B. (2016). Analysis of spironolactone polymorphs in active pharmaceutical ingredients and their effect on tablet dissolution profiles. Brazilian Journal of Pharmaceutical Sciences, 52, 613–621.
Wang, C. H., Meng, R., Wang, R., & Shen, Z. H. (2017). Synthesis and mechanism study of gelatin grafted acetone formaldehyde sulphonates as oil-well cement dispersant. Royal Society of Chemistry, 7, 31779–31788.
Sun, Z. J., Wu, L., Huang, W., Zhang, Z. L., Xili, L., Zheng, Y., Yang, B., & Dong, D. L. (2009). The influence of lactic on the properties of poly (glycerol-sebacate-lactic acid), Materials Science and Engineering C. Materials for Biological Applications, 29, 178–182.
Liang, S. L., Yang, X. Y., Fang, X. Y., Cook, W., Thouas, G. A., & Chen, Q. (2011). In vitro enzymatic degradation of poly (glycerol sebacate)-based materials. Journal of Biomaterials Applications, 32(84), 86–96.
Grossen, P., Witzigmann, D., Sieber, S., & Huwyler, J. (2017). PEG-PCL based nanomedicine: a biodegradable drug delivery system and its application. Journal of Control Release, 260, 46–60.
Wallace, D. G., & Rosenblatt, J. (2003). Collagen gel systems for sustained delivery and tissue engineering. Advanced Drug Delivery Reviewes, 55, 1631–1649.
Sahoo, N., Sahoo, R. K., Biswas, N., Guha, A., & Kuostu, K. (2015). Recent advancement of gelatin nanoparticles in drug and vaccine delivery. International Journal of Biological Macromolecules, 81(3), 17–31.
Sisson, A. L., Ekinci, D., & Lendlein, A. (2013). The contemporary role of Ɛ-caprolactone chemistry to create advanced polymer architectures. Polymer Journal, 54, 4333–4350.
Ding, L., Hao, C., Xue, Y., & Ju, H. (2007). A bio-inspired support of gold nanoparticles-chitosan nanocomposites gel for immobilization and electrochemical study of K562 leukemia cells. Journal of Biological Macromoles, 8, 1341–1346.
Woodruff, M. A., & Hutmacher, D. W. (2010). The return of a forgotten polymer-polycaprolactone in 21st century. Journal of Progress in Polymer Science, 35(12), 17–56.
Auffan, M., Rose, J., Bottero, J. Y., Lowry, G. V., Jolivet, J. P., & Wiesner, M. R. (2009). Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nature Nanotechnology, 4, 634–641.
Balfourier, A., Luciani, N., Wang, G., & Carn, F. (2019). Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Journal of Computational Biology, 117(1), 103–113.
Zimmermann, W. H., & Eschenhagen, T. (2003). Cardiac tissue engineering for replacement therapy. Heart Fail Reviews, 8, 259–269.
Gasperini, L., Mano, J. F., & Reis, R. L. (2014). Natural polymers for the microencapsulation of cells. Journal of Royal Society Interface, 11, 8–17.
Henson, J. C., Jensen, H., Balachandran, K., Rao, R., Kim, J. W., Jensen, M. (2020). Nanoenvironment: The Role of Nanomaterials in Stem Cell Differentiation and Stem Cell Tissue Engineering, Soft Materials Biomedical Materials, pp, 361–400.
Chandarana, M., Curtis, A., & Hoskins, C. (2018). The use of nanotechnology in cardiovascular disease. Journal of Applied Nanoscience, 8, 1607–1619.
Sajkiewicz, P., & Kolbuk, D. (2014). Electrospinning of gelatin for tissue engineering-molecular conformation as one of the overlooked problems. Journal of Biomaterials Science Polymer Edition, 25, 2009–2022.
Arun, A., Malrautu, P., Laha, A., & Ramakrishna, S. (2021). Gelatin nanofibers in drug delivery systems and tissue engineering. Journal Engineering Science, 16, 71–81.
Liu, W., Yang, X. L., & Winston, W. S. (2011). Preparation of uniform-sized multiple emulsions and micro/nano particulates for drug delivery by membrane emulsification. Journal Pharmaceutical Sciences, 100, 75–93.
Dorfman, J., Doung, M., Zibaitis, A., Pelletier, M. P., Tim, D., Li, C., & Chiu, R. C. (1998). Myocardial tissue engineering with autologous myoblast implantation. Journal of Thoracic and Cardiovascular Surgery, 116, 51–744.
Lal, A., Veinot, J. P., & Leenen, F. H. (2004). Critical role of CNS of aldosterone in cardiac remodeling post-myocardial infarction in rats. Cardiovascular Research, 64, 437–447.
Feiner, R., Fleischer, S., Shapira, A., Kalish, O. R., & Dvir, T. (2018). Multifunctional degradable electronic scaffolds for cardiac tissue engineering. Journal of Control Release, 281, 189–195.
Acknowledgements
The authors gratefully acknowledge the University of Isfahan and Isfahan University of Medical Sciences (Central Laboratory, School of Medicine) for their sincere contributions to this research.
Funding
This paper was not financially supported.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghaziof, S., Shojaei, S., Mehdikhani, M. et al. The Effect of Spironolactone Loading on the Properties of 3D-Printed Polycaprolactone/Gold Nanoparticles Composite Scaffolds for Myocardial Tissue Engineering. J Bionic Eng 21, 924–937 (2024). https://doi.org/10.1007/s42235-023-00458-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-023-00458-3