Abstract
Overdoses of pesticides lead to a decrease in the yield and quality of plants, such as beans. The unconscious use of deltamethrin, one of the synthetic insecticides, increases the amount of reactive oxygen species (ROS) by causing oxidative stress in plants. In this case, plants tolerate stress by activating the antioxidant defense mechanism and many genes. 5-Aminolevulinic acid (ALA) improves tolerance to stress by acting exogenously in low doses. There are many gene families that are effective in the regulation of this mechanism. In addition, one of the response mechanisms at the molecular level against environmental stressors in plants is retrotransposon movement. In this study, the expression levels of superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR), and stress-associated protein (SAP) genes were determined by Q-PCR in deltamethrin (0.5 ppm) and various doses (20, 40, and 80 mg/l) of ALA-treated bean seedlings. In addition, one of the response mechanisms at the molecular level against environmental stressors in plants is retrotransposon movement. It was determined that deltamethrin increased the expression of SOD (1.8-fold), GPX (1.4-fold), CAT (2.7-fold), and SAP (2.5-fold) genes, while 20 and 40 mg/l ALA gradually increased the expression of these genes at levels close to control, but 80 mg/l ALA increased the expression of these genes almost to the same level as deltamethrin (2.1-fold, 1.4-fold, 2.6-fold, and 2.6-fold in SOD, GPX, CAT, and SAP genes, respectively). In addition, retrotransposon-microsatellite amplified polymorphism (REMAP) was performed to determine the polymorphism caused by retrotransposon movements. While deltamethrin treatment has caused a decrease in genomic template stability (GTS) (27%), ALA treatments have prevented this decline. At doses of 20, 40, and 80 mg/L of ALA treatments, the GTS ratios were determined to be 96.8%, 74.6%, and 58.7%, respectively. Collectively, these findings demonstrated that ALA has the utility of alleviating pesticide stress effects on beans.
Similar content being viewed by others
Introduction
Dry bean is a warm-season plant that is rich in protein and vitamins and can easily grow in almost any type of soil. Also, it has a large cultivation area and is an important legume in terms of production (28.9 million tons, FAO 2019) among edible legumes in the world. However, the richness of the bean’s protein ratio increases its susceptibility to diseases and harmful insects (Mullins and Arjmandi 2021). In this sense, it becomes necessary to use modern agricultural techniques and inputs in order to increase the yield and quality of agricultural products and to combat diseases and pests.
The use of pesticides is a form of agricultural struggle in order to protect agricultural products from the damage of diseases, pests, and weeds. Deltamethrin [(S)-α-cyano-3-phenoxybenzyl (1R, 3R)-cis-2,2-dimethyl-3- (2,2-dibromovinyl)-2,2-cyclopropanecarboxylate] is a synthetic pyrethiroid and a broad-spectrum insecticide (Sayeed et al. 2003). It is known that deltametrin has been used successfully to control aphid infestation in fields where important crops such as beans are grown (Johnstone 1984). However, many sections of plants, such as cells (Mukhopadhyay et al. 2006), genomes (Chauhan et al. 2007; Ansari et al. 2009; Aylward et al. 2011), and chromosomes (Marques et al. 2014), are negatively affected due to the accumulation and non-degradation of insecticides by forming insoluble bonds in agricultural products (Bashir et al. 2007). In this case, many genes are activated to protect crops from the effects of pesticide stress (Kishimoto et al. 2002; Tian et al. 2013). The expression of antioxidant enzyme genes [superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), and glutathione reductase (GR)] increases in order to avoid the harmful effects of ROS, whose amount increases against oxidative stress that occurs during pesticide stress. Furthermore, stress-associated proteins (SAPs) are known as response factors to abiotic and biotic stresses and confer stress tolerance to plants (Wang et al. 2020).
One of the response mechanisms created at the molecular level against environmental stressors is retrotransposon movement in plants. Although they are inactive during normal growth and development, they are activated during stress, increase the mutation rate, and also cause methylation changes in the genome. It has been determined that Ttd1a retrotransposon activated when exposed to salt and light stress, located next to a resistance gene, and thus protected the wheat against stress (Woodrow et al. 2010). Retrotransposon-based markers have a key role in determining retrotransposon movements induced by stress. REMAP is one of these markers that amplify the DNA region between retrotransposon and simple sequence repeats (Kalendar et al. 2011).
Plants activate many plant growth regulators (PGRs) as well as antioxidant defense mechanisms, genes, and retrotransposon movements in order to tolerate damage to their metabolism. Also, the exogenous use of PGRs has a positive effect on increasing stress tolerance in stressed plants (Ali et al. 2013). One of these regulators, 5-aminolevulinic acid (ALA), is a precursor molecule involved in the biosynthesis of porphyrins such as chlorophyll (Chl), vitamin B12, and heme in plants (Balestrasse et al. 2010; Ali et al. 2013). The exogenous application of ALA is an effective antistress agent under optimum conditions that plays a role in the development of plant tolerance. It has been demonstrated in many stress studies, such as low temperature (Balestrasse et al. 2010), high temperature (Zhang et al. 2012), low light (Sun et al. 2009), excessive salinity (Naeem et al. 2011), heavy metal stress (Ali et al. 2013), and herbicide stress (Zhang et al. 2008).
In this study, we aimed to determine the expression levels of the SOD, CAT, GPX, and SAP genes, which are induced by the activation of the antioxidant mechanism against the oxidative damage caused by deltamethrin when used in excessive doses. Furthermore, it was assessed whether ALA, which has previously been shown to have a healing role in our earlier study (Taspinar et al. 2017), induced a change in the expression of these genes when combined with deltamethrin. Also, retrotransposon mobility and the rate of polymorphism were determined using the REMAP technique.
Material and methods
Plant material
Phaseolus vulgaris L. cv. Elkoca seeds were used as plant material provided by the Ataturk University Faculty of Agriculture.
Growth conditions, ALA, and deltamethrin treatments
The seeds used in the experiment were selected based on their equal sizes, sterilized with a 5% hypochlorite solution for 5 min, and rinsed three times with distilled water. Then, they were germinated in a hydroponic system at 25 °C for 16 light and 8 dark hours in plastic boxes (Arslan 2021) containing Hoagland solution (Sigma H2395-10 L) (Hoagland and Arnon 1938). By selecting from 7-day-old plants, 10 seedlings were obtained in an equivalent growth time. These seedlings were kept in the same conditions as the others. The experiment was conducted using a completely random design with three replications. ALA solutions (Sigma, A3785) [0 (control), 20, 40, and 80 mg/l] were sprayed on 20-day-old seedling leaves (Beyzaei et al. 2015). After 5 days of ALA treatment, a 0.5 ppm deltamethrin solution (Sigma, 45423) was sprayed on the leaves (Duran et al. 2015). Bulk sample strategy was applied for molecular analysis. Leaf samples were harvested 5 days after deltamethrin application from five randomly selected plants for each replication of treatments and were stored at − 80 °C.
Total RNA extraction, cDNA synthesis, and gene expression
Total RNA from 100 mg of leaves was extracted with the RNeasy Plant Mini Kit (Qiagen) according to the suppliers’ instructions. RNA purity and concentrations were assessed by determining the spectrophotometric absorbance of the samples with a NanoDrop-1000 spectrophotometer (OD 260/230>2). RNA integrity was evaluated on a 1.2% agarose gel, stained with ethidium bromide, and visualized with UV light. First-strand cDNA synthesis was performed with the RevertAid First-Strand cDNA Synthesis Kit (Thermo Scientific) as described by Arslan et al. (2021). The quantitative PCR was performed with the SYBR Green/ROX qPCR Kit (Thermo Scientific) according to the manufacturer’s protocol, and the following genes were amplified: SOD, GPX, CAT, and SAP. β-Actin was preferred as a housekeeping gene. To design the primers for genes, databases related to bioinformatic studies conducted in the Phaseolus vulgaris genome were used (Table 1). Accession numbers for genes were found using the Pfam (pfam.xfam.org) database. Then, the Phytozom (https://phytozome.jgi.doe.gov/) database, which is the plant genomic source, was used. Finally, primers were created using the Primer3 (http://frodo.wi.mit.edu/) program from selected base sequences. The Q-PCR reactions were run in a Qiagen Rotor-Gene, and the cycling conditions consisted of initial denaturation at 95 °C for 10 min, followed by 40 cycles of amplification at 95 °C for 15 s, 56–65 °C for 30 s, and extension at 72 °C for 30 s. The relative gene expression levels, which were determined using the 2−ΔΔCt equation, were calculated to get the expression fold change (Livak and Schmittgen 2001). Each sample was analyzed in three technical replicates. A one-way ANOVA was performed to evaluate the effect of treatments on gene expression. Duncan’s multiple range test (P ≤ 0.05) was performed to compare the mean values. The data were analyzed using SAS 9.3 software for Windows.
Genomic DNA extraction
Total DNA was extracted from 0.1 g of leaves from each treated group by the CTAB method of Shams et al. (2020). Integrity and quality of DNA were evaluated by electrophoresis on a 1% agarose gel.
REMAP
The REMAP reactions were based on a previously published method (Yigider et al. 2020). For amplification, the IRAP primers [Nikita-E2647, Stowaway, Sukkula, and Bare 1(0)] were combined with ISSR primers (8081, 8082) (Table 2). Amplifications were carried out in a Thermo Scientific™ Arktik™ Thermal Cycler with the following PCR programs: an initial denaturation at 95 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, distinct temperatures for each primer for 1 min, and 72 °C for 2 min; and a final extension at 72 °C for 15 min. PCR products were run by electrophoresis on 2% agarose gel in 0.5× TBE buffer stained with ethidium bromide and photographed in the DNR Minibis Gel Documentation System (USA).
REMAP data analysis
The gel images obtained were evaluated with the TotalLab TL120 program. Genomic template stability (GTS) (%) for each primer was calculated using the formula 100 − (100 × a/n) according to Atienzar et al. (1999). “a” in the formula indicates the REMAP polymorphic profiles determined for each treated sample, and “n” indicates the total number of DNA bands obtained in the negative control group with the relevant primer. The polymorphism observed in the REMAP profiles of the treatment groups includes the emergence of a new band or the disappearance of an existing band compared to the negative control group. For all treatments, a binary matrix was generated based scored as 1 (present) or 0 (absent) for each primer. The following calculations were carried out with the use of NTSYSpc 2.11f software. The Jaccard’s similarity coefficient was calculated by using the SIMQUAL module. The similarity coefficients were then used to construct dendrograms, by using the UPGMA (unweighted pair group method with arithmetic averages) employing the SAHN. The goodness of dendongram was verified in the MXCOMP program by using Jacard’s similarity matrix and co-phenetic value matrix. The three-dimensional PCoA was performed based on the similarity matrix.
Results and discussion
Gene expression profile of some antioxidant enzymes and stress protein under deltamethrin and ALA treatments
Unfavorable environmental conditions and insect infestations are the strongest factors limiting yield in beans (Gogo et al. 2014). Pesticides, such as deltamethrin, are frequently used in agricultural lands to reduce the effects of harmful organisms. Overdose of deltamethrin causes oxidative damage in plants by activating ROS (Bashir et al. 2007). ROS play a dual role in plant responses to abiotic stress, both as toxic by-products of stress metabolism and as an important signal transduction molecule in complex metabolic processes responsible for the emergence of stress responses based on calcium, hormone, and protein phosphorylation (Miller et al. 2010). The uncontrolled oxidation obtained when ROS are overproduced leads to cellular damage and ultimately cell death. In order to prevent the plant from being damaged by this situation, the current antioxidant mechanism should keep active oxygen under control (Bose et al. 2014). When these removal mechanisms are not damaged by stress, ROS are rapidly destroyed by antioxidant mechanisms (Ahmad et al. 2010). Among the regions targeted by ROS, such as proteins and DNA, that are difficult to repair result in genetic damage. Genetic studies in seedlings of Phaseolus vulgaris also suggest a link between pesticide stress and oxidative stress, since deltamethrin induces the expression of several genes that are also induced by oxidative stress (Ajermoun et al. 2022; Boulahia et al. 2023). In this study, expression levels of the SOD, CAT, and GPX genes, which are antioxidant genes, were determined. According to our results, all of these genes were upregulated by deltamethrin stress.
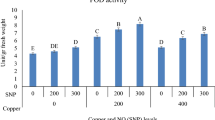
SOD is the first line of defense against ROS during abiotic stress in a plant cell. Control of SODs in both expression and activity of ROS contributes to the regulation of stress tolerance (Forman 2007; Liu et al. 2008). According to the results of the SOD gene expression analysis, the rate of gene expression in deltamethrin treatment alone was about twofold higher than that of the control (1.8) (Fig. 1a). Similarly, GPX and CAT genes were upregulated in deltamethrin treatment approximately two and threefold (1.4 and 2.7) according to non-treated seedlings, respectively (Fig. 1b, c). To scavenge the ROS efficiently, the activity of APX and SOD must be high to remove the H2O2 produced by superoxide ion dismutation (Pospíšil 2012). Therefore, in our study, the high expression levels of both SOD, CAT, and GPX genes could be responsible for the removal of ROS. In a similar study, Sharma et al. (2015) investigated the expression levels of CAT, SOD, APX, and GR enzyme genes in salt and pesticide stress applied to rice and found that all genes were highly upregulated in both stresses.
The expression patterns of a SOD, b GPX, c CAT, and d SAP genes under 5-aminolevulinic acid (ALA) and deltamethrin (DM) treatments. Data represent the means ±SD of three replications. The different letter on the graph indicates significant differences based on Duncan’s multiple range test (P ≤ 0.05)
As well as antioxidant genes, the SAP gene was highly upregulated (2.5) in deltamethrin stress (Fig. 1d). Proteins in the SAP family contain the A20/AN1 zinc finger domain and are known to be important determinants of stress responses in plants (Vij and Tyagi 2006). Similar results were obtained in different plants during different abiotic stresses, indicating that SAP genes (OSISAP) found in rice in particular are induced by abiotic stress (Vij and Tyagi 2006). Overexpression of the OSISAP1 gene in tobacco (Nicotiana tabacum) increases tolerance to cold, drought, and salt stress (Mukhopadhyay et al. 2004). Similarly, the OSISAP8 gene, which is transferred to the tobacco plant, is thought to have a role in the development of tolerance against abiotic stress (Kanneganti and Gupta 2008). Giri et al. (2011) determined that the OSISAP11 gene transferred to transgenic Arabidopsis interacts with OSIRLCK253, a receptor-like cytoplasmic kinase, providing tolerance to drought and salt stress, as well as the TaSAP5 gene in wheat (Zhang et al. 2017). Studies have associated SAP proteins with roles such as ubiquitination, redox detection, and regulation of gene expression under abiotic stress (Ströher et al. 2009; Ben-Saad et al. 2012; Kang et al. 2013). However, the mechanism by which SAP proteins play the main role mechanically in stress responses has not been fully elucidated.
In recent studies, plant growth regulators have also been reported to have roles in the regulation of the plant defense system against various stresses (Zhang et al. 2008; Balestrasse et al. 2010; Naeem et al. 2011; Zhang et al. 2012; Ali et al. 2013). In addition, it has been proven that high concentrations of ALA play a role as an herbicide or insecticide (Chon 2003). As a matter of fact, in our study, ALA in low doses (20 and 40 mg/l) was beneficial in creating stress tolerance by increasing the expression of antioxidant genes. In the 80 mg/l ALA application, it was determined that SOD gene expression was higher than in the delthametrin treatment, and the difference between these two treatments had been significant (Fig. 1a). On the other hand, CAT and GPX gene expressions in 80 mg/l ALA application were close to the results obtained from deltamethrin application (Fig. 1b, c). In addition, when ALA and deltamethrin were treated together, 40 mg/l of ALA increased the expression of these genes more. This situation may be related to ALA protecting the cell against the destructive effect of ROS by removing H2O2 (Ali et al. 2015). Sharma et al. (2015) found that the SOD gene was more induced in brassinosteroid applications than salt or pesticide stress alone in rice. Exogenously applied high concentrations of ALA accumulate in excessive amounts in cells, causing an increase in the amount of ROS by being exposed to both enolization and aerobic oxidation with metal catalysis (Reyter and Tyrrel 2000). In this case, the enzyme called heme oxygenase degrades the free heme group, converts bilirubin into iron and carbon monoxide (Shekhawat and Verma 2010), and causes a decrease in oxidative stress in the plant (Grochot-Przeczek et al. 2012). While the mechanism for oxidative stress and degradation of the heme group remains unclear, it is thought to be an evolutionary protection mechanism given by the plant cell to counteract the destructive effect of the free heme group (Kumar and Bandyopadhyay 2005). Noriega et al. (2012) determined that the cadmium increased the ALA content in the root, leaf, and nodule parts of the soybean, and the plant was exposed to more oxidative stress. At the same time, it was found that cadmium or ALA applications both inhibited antioxidant enzyme activities and caused a significant decrease in SOD and guaicole peroxidase expression.
Our experiment results also indicated that SAP gene was gradually upregulated in ALA treatments. However, the highest expression rate was 80 mg/l ALA + deltamethrin and the lowest was in the application of 20 mg/l ALA. ZFP185, a A20/AN1 zinc finger protein, is linked to abscisic acid and gibberellic acid, which regulate the cell growth and stress response mechanism (Zhang et al. 2016). Thus, it can be assumed that the SAP gene works in conjunction with these hormones to establish stress tolerance. In our study, it is thought that 80 mg/l ALA application has a more damaging effect on deltamethrin by acting as an insecticide; it may have a role in the formation of stress tolerance by linking with signal molecules such as abscisic acid and gibberellic acid, which leads to a greater increase in the expression of these genes.
Changes in REMAP pattern under deltamethrin and ALA treatments
Another effect of various environmental stressors at the genome level is retrotransposon mobility. In our study, the retrotransposon polymorphism caused by deltamethrin was determined by REMAP. A total of eight primer pairs were tried for REMAP analysis, and 113 bands were obtained, and 98 of them were determined to be polymorphic bands. All of the primers were determined to be polymorphic. Maximum number of polymorphic bands counted in Bare 1 (0) + ISSR 8081 and minimum in Stowaway + ISSR 8081 primer pairs (Table 3). The polymorphic information content (PIC) value of the primer pairs used to determine the molecular effects of the treatments varied between 0.365 and 0.427, and the average was 0.382 (Table 3). The maximum PIC value for dominant markers is 0.5. Because two alleles are assumed per locus, both are affected by the number and frequency of alleles. In this respect, the Stowaway + ISSR 8081 primer pair had the highest PIC value (Table 3). On the other hand, the discriminating power (D) parameter used in the evaluation of the primers shows the efficiency of the primers in the identification of individuals. The D value of the primers varied between 0.408 and 0.867 (Table 3). The Bare 1 (0) + ISSR 8081 primer pair, which has both the discrimination power and the highest polymorphic band content, was determined to be the most distinctive primers.
With the treatment of deltamethrin, a 73% polymorphism ratio occurred. When examining the effects of applications in terms of GTS ratio, the lowest GTS value was obtained in deltamethrin-treated seedlings compared to the control. While the molecular weights of the missing bands of applications compared to the control are between 50 and 1440 bp, the newly formed bands were between 224 and 1581 bp (Table 4). When the effects of different doses of ALA were examined compared to the control, it was determined that 40 and 80 mg/L ALA doses caused a decrease in the GTS values, depending on the dose increase. On the other hand, 40 mg/L ALA reduced the negative effect of deltamethrin on GTS (Fig. 2).
The similarity index of the applications varied between 0.38 and 0.73. The highest similarity with the control occurred between 20 mg/L ALA, while the least similarity was deltamethrin treatment (Table 5). This result showed that deltamethrin caused a significant change induced by retrotransposons in the genome. On the other hand, when the similarity indexes of ALA applications against deltamethrin applications with control were evaluated, it was determined that 40 mg/L ALA applications against deltamethrin had the highest similarity between these applications with control (Table 5). Furthermore, genetic similarity values were used for cluster analysis through UPGMA, resulting in a dendrogram (Fig. 3). The cophenetic correlation coefficient was calculated to evaluate the goodness of the dedongram. This value was determined as 0.84 and indicated a good fit (Rholf 1993). The UPGMA analysis clearly indicated differences among treatments. The treatments were grouped into five clusters, with cluster I containng control and 20 mg/L ALA, cluster II containing 80 mg/L ALA and 80 mg/L ALA + DM, cluster III containing 40 mg/L ALA, cluster IV containing DM, and cluster V containing 20 mg/l ALA + DM and 80 mg/L ALA + DM (Fig. 3). The results of PCoA support the results obtained from cluster analysis obtained through UPGMA (Fig. 4). While there are many studies on increasing retrotransposon mobility and polymorphism with stress, there is no literature about deltamethrin stress. Evrensel et al. (2011) investigated the mobility of Nikita and BARE-1 retrotransposons in barley (Hordeum vulgare L.) under plant tissue culture conditions using the IRAP molecular marker technique and reported that the polymorphism that occurs in callus of different ages is due to the movements of Nikita and BARE-1 retrotransposons. Yigider et al. (2020) determined the polymorphism resulting from the movement of some retrotransposons by heavy metal stress in maize using IRAP and REMAP techniques. In our previous study, where we determined the effect of deltamethrin and ALA applications on DNA methylation changes (Taspinar et al. 2017), the high level of DNA methylation polymorphism caused by deltamethrin decreased to lower values with ALA. Furthermore, a change in the GTS rate was observed at all doses of ALA in this study. This may be due to epigenetic change. Taspinar et al. (2017) indicated that ALA caused changes in DNA methylation in Phaseolus vulgaris. Reinders et al. (2009) and Mirouze and Paszkowski (2011) reported that epigenetic situation changes may promote the movement of DNA transposons and retroelements, which are abundant in the plant genome.
Conclusion
Overall, our results unequivocally established that SOD, CAT, GPX, and SAP genes are induced by the activation of the antioxidant mechanism against the oxidative damage caused by deltamethrin. In addition, it was determined that ALA caused a change in the expression of these genes when applied together with deltamethrin. Thus, an important step of the plant’s response mechanism against stress has been elucidated. At the same time, the retrotransposon mobility caused by deltamethrin stress and the effect of ALA on this mobility and its polymorphism ratio were determined using the REMAP technique. In this respect, it is thought that this is the first study conducted on this subject, and the results obtained as a result of this study will lead to the cultivation of the bean plant, which has an important place in the world, on lands exposed to intense pesticide stress and lead to other studies in this field.
References
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and non enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161–175. https://doi.org/10.3109/07388550903524243
Ajermoun N, Aghris S, Ettadili F, Alaoui OT, Laghrib F, Farahi A, Lahrich S, Bakasse M, Saqrane S, El Mhammedi MA (2022) Phytotoxic effect of the insecticide imidacloprid in Phaseolus vulgaris L. plant and evaluation of its bioaccumulation and translocation by electrochemical methods. Environ Res 214:113794. https://doi.org/10.1016/j.envres.2022.113794
Ali B, Tao QJ, Zhou YF, Gill RA, Ali S, Rafiq MT (2013) 5-Aminolevolinic acid mitigates the cadmium-induced changes in Brassica napus as revealed by the biochemical and ultra-structural evaluation of roots. Ecotoxicol Environ Saf 92:271–280. https://doi.org/10.1016/j.ecoenv.2013.02.006
Ali B, Gill RA, Yang S, Gill MB, Farooq MA, Liu D, Daud MK, Ali S, Zhou W (2015) Regulation of cadmium-induced proteomic and metabolic changes by 5- aminolevulinic acid in leaves of Brassica napus L. PLoS One 10:e0123328. https://doi.org/10.1371/journal.pone.0123328
Ansari RA, Kaur M, Ahmad F, Rahman S, Rashid H, Islam F, Raisuddin S (2009) Genotoxic and oxidative stress-inducing effects of deltamethrin in the erythrocytes of a fresh water biomarker fish species, Channa punctata Bloch. Environ Toxicol 24:429–436. https://doi.org/10.1002/tox.20445
Arslan E, Agar G, Aydın M (2021) Humic acid as a biostimulant in ımproving drought tolerance in wheat: the expression patterns of drought-related genes. Plant Mol Biol Report 39:508–519. https://doi.org/10.1007/s11105-020-01266-3
Atienzar FA, Conradi M, Evenden AJ, Jha AN, Depledge MH (1999) Qualitative assessment of genotoxicity using random amplified polymorphic DNA: comparison of genomic template stability with key fitness parameters in Daphnia magna exposed to benzo [a] pyrene. Environ Toxicol Chem 18:2275–2282. https://doi.org/10.1897/1551-5028(1999)018<2275:qaogur>2.3.co;2
Aylward LL, Krishnan K, Kirman CR, Nong A, Hays SM (2011) Biomonitoring equivalents for deltamethrin. Regul Toxicol Pharmacol 60:189–199. https://doi.org/10.1016/j.yrtph.2011.03.014
Balestrasse KB, Tomaro ML, Batlle A, Noriega GO (2010) The role of 5- aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 71:2038–2045. https://doi.org/10.1016/j.phytochem.2010.07.012
Bashir F, Siddiqi MTO, Iqbal M (2007) The antioxidative response system in Glycine max (L) Merr exposed to deltamethrin, a synthetic pyrethroid insecticide. Environ Pollut 147:94–100. https://doi.org/10.1016/j.envpol.2006.08.013
Ben-Saad R, Ben-Ramdhan W, Zouari N, Azaza J, Mieulet D, Guiderdoni E, Ellouz R, Hassairi A (2012) Marker-free transgenic durum wheat cv Karim expressing the AlSAP gene exhibits a high level of tolerance to salinity and dehydration stresses. Mol Breed 30:521–533. https://doi.org/10.1007/s11032-011-9641-3
Beyzaei Z, Averina NG, Sherbakov RA (2015) Involvement of nitrate reductase in the ameliorating effect of 5-aminolevulinic acid on NaCl-stressed barley seedlings. Acta Physiol Plant 37:1–8. https://doi.org/10.1007/s11738-014-1752-0
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257. https://doi.org/10.1093/jxb/ert430
Botstein D, White RL, Skolnick M, Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet 32(3):314. https://doi.org/10.31274/rtd-180813-11683
Boulahia K, Ould said C, Abrous-Belbachir O (2023) Exogenous application of salicylic acid improve growth and some physio-biochemical parameters in herbicide stressed Phaseolus vulgaris L. Gesunde Pflanz 75:2301–2318. https://doi.org/10.1007/s10343-023-00878-5
Chauhan LK, Kumar M, Paul BN, Goel SK, Gupta SK (2007) Cytogenetic effects of commercial formulations of deltamethrin and/or isoproturon on human peripheral lymphocytes and mouse bone marrow cells. Environ Mol Mutagen 48:636–643. https://doi.org/10.1002/em.20330
Chon SU (2003) Herbicidal activity of delta-aminolevulinic acid on several plants as affected by application methods. Korean J Crop Sci 48(1):50–55
Duran RE, Kilic S, Coskun Y (2015) Response of maize (Zea mays L saccharata Sturt) to different concentration treatments of deltamethrin. Pestic Biochem Physiol 124:15–20. https://doi.org/10.1016/j.pestbp.2015.03.011
Evrensel C, Yilmaz S, Temel A, Gozukirmizi N (2011) Variations in BARE-1 insertion patterns in barley callus cultures. Genet Mol Res 10:980–987. https://doi.org/10.4238/vol10-2gmr965
FAO (2019) Food outlook. In: Biannual Report on Global Food Markets http://www.fao.org/3/ca6911en/ca6911en.pdf. Accessed 22 March 2023
Forman HJ (2007) Use and abuse of exogenous H2O2 in studies of signal transduction. Free Radic Biol Med 42:926–932. https://doi.org/10.1016/j.freeradbiomed.2007.01.011
Giri J, Vij S, Dansana PK, Tyagi AK (2011) Rice A20/AN1 zinc-finger containing stress-associated proteins (SAP1/11) and a receptor-like cytoplasmic kinase (OsRLCK253) interact via A20 zinc-finger and confer abiotic stress tolerance in transgenic Arabidopsis plants. New Phytol 191:721–732. https://doi.org/10.1111/j.1469-8137.2011.03740.x
Gogo EO, Saidi M, Ochieng JM, Martin T, Baird V, Ngouajio M (2014) Microclimate modification and insect pest exclusion using agronet improve podyield and quality of French bean. Hortscience 49:1298–1304. https://doi.org/10.21273/hortsci.49.10.1298
Grochot-Przeczek A, Dulak J, Jozkowicz A (2012) Haem oxygenase-1: noncanonical roles in physiology and pathology. Clin Sci 122:93–103. https://doi.org/10.1042/cs20110147
Hoagland DR, Arnon DI (1938) Growing plants without soil by the water-culture method. Circ Calif Agric Exp Stn 1938:16
Johnstone GR (1984) Control of primary infections of subterranean clover red leaf virus, a luteovirus, in a broad bean crop with the synthetic pyrethroid deltamethrin Australas. Plant Pathol 13:55–56. https://doi.org/10.1071/app9840055
Kalendar R, Flavell AJ, Ellis THN, Sjakste T, Mois C, Schulman AH (2011) Analysis of plant diversity with retrotransposon-based molecular markers. Heredity 106:520–530. https://doi.org/10.1038/hdy.2010.93
Kang M, Abdelmageed H, Lee S, Reichert A, Mysore KS, Allen RD (2013) AtMBP-1, an alternative translation product of LOS2, affects abscisic acid responses and is modulated by the E3 ubiquitin ligase AtSAP5. Plant J 76:481–493. https://doi.org/10.1111/tpj.12312
Kanneganti V, Gupta AK (2008) Overexpression of OsiSAP8, a member of stress associated protein (SAP) gene family of rice confers tolerance to salt, drought and cold stress in transgenic tobacco and rice. Plant Mol Biol 66:445–462. https://doi.org/10.1007/s11103-007-9284-2
Kishimoto K, Nishizawa Y, Tabei Y, Hibi T, Nakajima M, Akutsu K (2002) Detailed analysis of rice chitinase gene expression in transgenic cucumber plants showing different levels of disease resistance to gray mold (Botrytis cinerea). Plant Sci 162:655–662. https://doi.org/10.1016/s0168-9452(01)00602-1
Kumar S, Bandyopadhyay U (2005) Free heme toxicity and its detoxification systems in human. Toxicol Lett 157:175–188. https://doi.org/10.1016/j.toxlet.2005.03.004
Liu B, Chen Y, StClair DK (2008) ROS and p53: a versatile partnership. Free Radic Biol Med 44:1529–1535. https://doi.org/10.1016/j.freeradbiomed.2008.01.011
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2:402–408. https://doi.org/10.1006/meth.2001.1262
Marques A, Custodio M, Guilherme S, Gaivao I, Santos MA, Pacheco M (2014) Assessment of chromosomal damage induced by a deltamethrin-based 41 insecticide in fish (Anguilla anguilla L) e a follow-up study upon exposure and postexposure periods. Pestic Biochem Physiol 113:40–46. https://doi.org/10.1016/j.pestbp.2014.06.003
Miller GAD, Suzuki N, Ciftci-Yilmaz S, Mittler RON (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mirouze M, Paszkowski J (2011) Epigenetic contribution to stress adaptation in plants. Curr Opin Plant Biol 14:267–274. https://doi.org/10.1016/j.pbi.2011.03.004
Mukhopadhyay A, Vij S, Tyagi AK (2004) Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc Natl Acad Sci U S A 101:6309–6314. https://doi.org/10.1073/pnas.0401572101
Mukhopadhyay I, Siddique HR, Bajpai VK, Saxena DK, Chowdhuri DK (2006) Synthetic pyrethroid cypermethrin induced cellular damage in reproductive tissues of Drosophila melanogaster: Hsp70 as a marker of cellular damage. Arch Environ Contam Toxicol 51:673–680. https://doi.org/10.1007/s00244-005-0169-6
Mullins AP, Arjmandi BH (2021) Health benefits of plant-based nutrition: focus on beans in cardiometabolic diseases. Nutrients 13(2):519. https://doi.org/10.3390/nu13020519
Naeem MS, Rasheed M, Liu D, Jin ZL, Ming DF, Yoneyama K (2011) 5- Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta Physiol Plant 33:517–528. https://doi.org/10.1007/s11738-010-0575-x
Noriega G, Caggiano E, Lecube ML, Santa Cruz D, Batlle A, Tomaro M, Balestrasse KB (2012) The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 25:1155–1165. https://doi.org/10.1007/s10534-012-9577-z
Pospíšil P (2012) Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim Biophys Acta 1817:218–231. https://doi.org/10.1016/j.bbabio.2011.05.017
Prevost A, Wilkinson MJ (1999) A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98:107–112. https://doi.org/10.1007/s001220051046
Reinders J, Wulff BB, Mirouze M, Marí-Ordóñez A, Dapp M, Rozhon W, Paszkowski J (2009) Compromised stability of DNA methylation and transposon immobilization in mosaic Arabidopsis epigenomes. Genes Dev 23:939–950. https://doi.org/10.1101/gad.524609
Reyter SW, Tyrrel RM (2000) The heme synthesis and degradation pathways: role in oxidant sensitivity Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28:289–309. https://doi.org/10.1016/s0891-5849(99)00223-3
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56:295–301. https://doi.org/10.1016/s0147-6513(03)00009-5
Shams M, Yildirim E, Arslan E, Agar G (2020) Salinity induced alteration in DNA methylation pattern, enzyme activity, nutrient uptake and H2O2 content in pepper (Capsicum annuum L.) cultivars. Acta Physiol Plant 42:59. https://doi.org/10.1007/s11738-020-03053-9
Sharma I, Bhardwaj R, Pati PK (2015) Exogenous application of 28- homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J Plant Growth Regul 34:509–518. https://doi.org/10.1007/s00344-015-9486-9
Shekhawat GS, Verma K (2010) Haem oxygenase (HO): an overlooked enzyme of plant metabolism and defence. J Exp Bot 61:2255–2270. https://doi.org/10.1093/jxb/erq074
Ströher E, Wang XJ, Roloff N, Klein P, Husemann A, Dietz KJ (2009) Redoxdependent regulation of the stress-induced zinc-finger protein SAP12 in Arabidopsis thaliana. Mol Plant 2:357–367. https://doi.org/10.1093/mp/ssn084
Sun YP, Zhang ZP, Wang LJ (2009) Promotion of 5-aminolevulinic acid treatment on leaf photosynthesis is related with increase of antioxidant enzyme activity in watermelon seedlings grown under shade condition. Photosynthetica 47:347–354. https://doi.org/10.1007/s11099-009-0055-y
Taspinar MS, Aydin M, Arslan E, Yaprak M, Agar G (2017) 5-Aminolevulinic acid improves DNA damage and DNA Methylation changes in deltamethrin-exposed Phaseolus vulgaris seedlings. Plant Physiol Biochem 118:267–273. https://doi.org/10.1016/j.plaphy.2017.06.026
Tian DQ, Pan XY, Yu YM, Wang WY, Zhang F, Ge YY, Shen XL, Shen FQ, Liu XJ (2013) De novo characterization of the Anthurium transcriptome and analysis of its digital gene expression under cold stress. BMC Genomics 14:827. https://doi.org/10.1186/1471-2164-14-827
Vij S, Tyagi AK (2006) Genome-wide analysis of the stress associated protein (SAP) gene family containing A20/AN1 zinc-finger(s) in rice and their phylogenetic relationship with Arabidopsis. Mol Gen Genomics 276:565–575. https://doi.org/10.1007/s00438-006-0165-1
Wang Z, Kuang J, Han B, Chen S, Liu A (2020) Genomic characterization and expression profiles of stress-associated proteins (SAPs) in castor bean (Ricinus communis). Plant Divers 43(2):152–162. https://doi.org/10.1016/j.pld.2020.07.010
Woodrow P, Pontecorvo G, Fantaccione S, Fuggi A, Kafantaris I, Carillo P (2010) Polymorphism of a new Ty1-copia retrotransposon in durum wheat under salt and light stresses. Theor Appl Genet 121:311–322. https://doi.org/10.1007/s00122-010-1311-z
Yigider E, Taspinar MS, Aydin M, Agar G (2020) Humic acid effects on retrotransposon polymorphisms caused by zinc and iron in the maize (Zea mays L) genome. Cereal Res Commun 49:193–198. https://doi.org/10.1007/s42976-020-00111-3
Zhang J, Li DM, Gao Y, Yu B, Xia CX, Bai JG (2012) Pretreatment with 5-aminolevulinic acid mitigates heat stress of cucumber leaves. Biol Plant 56:780–784. https://doi.org/10.1007/s10535-012-0136-9
Zhang N, Yin Y, Liu X, Tong S, Xing J, Zhang Y, Hu Z (2017) The E3 ligase TaSAP5 alters drought stress responses by promoting the degradation of DRIP proteins. Plant Physiol 175:1878–1892. https://doi.org/10.1104/pp.17.01319
Zhang WF, Zhang F, Raziuddin R, Gong HJ, Yang ZM, Lu L et al (2008) Effects of 5- aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J Plant Growth Regul 27:159–169. https://doi.org/10.1007/s00344-008-9042-y
Zhang Y, Lan H, Shao Q, Wang R, Chen H, Tang H, Zhang H, Huang J (2016) An A20/AN1-type zinc finger protein modulates gibberellins and abscisic acid contents and increases sensitivity to abiotic stress in rice (Oryza sativa). J Exp Bot 67:315–326. https://doi.org/10.1093/jxb/erv464
Acknowledgements
The authors are grateful to the Atatürk University, Scientific Research Projects (FBA-2018-6712).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was funded by Atatürk University, Scientific Research Projects (grant number FBA-2018-6712).
Author information
Authors and Affiliations
Contributions
Murat Aydin analyzed all statistic materials. Esra Arslan Yuksel performed the analyses. Mahmut Sinan Taspinar and Guleray Agar contributed in writing manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Peter Nick
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuksel, E.A., Aydin, M., Agar, G. et al. 5-Aminolevulinic acid treatment mitigates pesticide stress in bean seedlings by regulating stress-related gene expression and retrotransposon movements. Protoplasma 261, 581–592 (2024). https://doi.org/10.1007/s00709-023-01924-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-023-01924-9