Abstract
The global textile fiber output increased five times from 1975 to 2020. Also, in 2010, the combined demand for man-made and natural fibers was projected to increase by 84% within 20 years. Clothing materials are largely made from cotton or petroleum-based synthetic fibers; both sources, however, have adverse environmental impacts. Thus, cotton requires vast amounts of land, water, fertilizers and pesticides, and synthetic fibers are not biodegradable. This scenario has raised the need for further exploration of cellulose polymers as sustainable sources for the textile industry. Cellulose, the most abundant renewable organic material on earth, is an outstanding polymer that by chemical derivatization or modification can offer a broad range of applications. Dissolving-grade pulp (DGP), which consists of highly pure cellulose, is the most suitable material for manufacturing cellulose derivatives and regenerated fibers. The latter are typically obtained by using the viscose process, which has considerable adverse environmental impacts. Although the textile industry has progressed substantially, further efforts are still needed to make its entire production chain more sustainable. This article provides an in-depth introduction to the potential of fibers with a high cellulose content, known as dissolving-grade pulps. It reviews the properties of DGP, the cooking and purifying methods typically used to obtain it, and the process by which paper-grade pulp can be converted into dissolving-grade pulp. Also, it discusses traditional and recently developed technologies for producing regenerated cellulose fibers. Finally, it examines the potential for recovering cellulose from textile waste as a novel sustainable practice.
Similar content being viewed by others
Introduction
The textile and fashion industry constitutes one of the largest industrial sectors (Van Woensel and Lipp 2020). According to the Forests for Fashion report (UNECE/FAO 2019), the global fashion industry is valued at over 2.4 trillion dollars, employing more than 75 million people worldwide. However, the environmental footprint of the clothing life cycle remains controversial. Some studies indicate that textile industry contributes to 8–10% of global greenhouse gas (GHG) emissions and accounts for 20% of all wastewater pollution (de Oliveira et al. 2021); others, however, suggest smaller contribution, near 2–3% of GHG emissions on a global scale and 4–5% in European households (Sohn et al. 2021). Despite these controversial statistics, textile industry certainly has a high environmental impact, not only through the cultivation of crops and raw materials, but also through the manufacturing processes used to spin raw material into yarns, weave them into fabrics and apply finishing and chemical treatments to the fabrics. In fact, these processes consume large amounts of water and chemicals, are energy intensive, and contribute to carbon emissions. According to the Pulse of the Fashion Industry report (Kerr and Landry 2017), the global textile and clothing industry used 79 billion cubic meters of water in 2015, while the overall needs of the EU's economy amounted to 266 billion cubic meters in 2017 (Šajn 2019).
The “fast fashion” business model, based on rapid changes in clothing lines and trends, has further intensified the problem by promoting irresponsible consumption that leads to vast amounts of underused materials being discarded each year. Also, this phenomenon has promoted the use of low-cost synthetic fibers and shortened the lifespan of clothing as a result.
Approximately 24 million metric tons of textile fibers were produced worldwide in 1975. That amount increased nearly five times by 2020, where it reached 108 million metric tons (Statista) (Fig. 1). This increase in demand is explained by population growth, improved standard of living and the “fast fashion” phenomena. The latest forecast suggests that total fiber demand, including natural fibers, is going to increase from 72.5 million tons in 2010 to about 133–156 million tons in 2030, in parallel with an increase in per capita consumption from 10.5 to 15.5–17 kg per person per year (i.e., an average annual growth rate of 3.1%) (Bywater 2011; Hämmerle 2011; Sixta et al. 2013). According to European Environment Agency (2019), the amount of clothes per person bought in the EU increased by 40% from 1996 to 2012, associated with a fall in prices and a shortening in clothing life. As reported in Textiles and the environment: the role of design in Europe’s circular economy (European Environment Agency 2022), the EU-27 produced 6.9 million tons of finished textile products in 2020 and used 6.6 million tons of textiles. Another 8.7 million tons of finished textile products were imported from China, Bangladesh, and Turkey, mainly (European Environment Agency 2019).
Evolution of the global output of textile fibers and chemical fibers, which include synthetic fibers such as polyesters or polyamides, and man-made cellulose fibers such as viscose or rayon. Data from Statista. https://www.statista.com/statistics/263154/worldwide-production-volume-of-textile-fibers-since-1975/. Accessed February 2023
Textile fibers can be Natural or Man-Made Fibers (MMF) (Fig. 2). Natural fibers are classified as plant-based, animal or mineral according to their origin. All three types are renewable, biodegradable, lightweight, strong, and mechanically recyclable. According to the report Mapping the clothing impacts in Europe: the environmental cost by European Clothing Action Plan (Gray 2017), cotton accounts for 90% of all natural fibers and more than 43% of all clothing fibers used in the EU. However, cotton is considered especially problematic because it requires vast amounts of land, water, fertilizers and pesticides, although organic cotton can drastically reduce the environmental impacts as it requires less water (Pepper 2017). However, since the textile consumption is expected to continue increase annually, cotton cultivation will not be able to cover the future demands and create a “cellulose-gap” by 2030 as a result (Hämmerle 2011).

Adapted from Sinclair (2015)
Classification of textile fibers and examples of various types of fibers. Natural fibers can be divided into vegetable, mineral and animal or protein. Man-made fibers can be classified as regenerated fibers, synthetic fibers and inorganic fibers.
Man-made synthetic fibers currently dominate the textile market with around 70 million tons (nearly 63% of the global fiber production) in 2019 (Exchange 2020; Mendes et al. 2021). The most common synthetic fibers, which are made from fossil fuels and are not biodegradable, include polyester or polyethylene terephthalate (PET) with 16% of all fibers used in clothes, polyamide (often called nylon); acrylic and modacrylic; polypropylene; polyethylene; elastanes (known as spandex in the USA) and speciality high-tenacity fibers such as the high-performance aramids and UHMwPE (Ultra High Molecular Weight PolyEthylene) (Gray 2017). These fibers have smaller water footprint, since they require less water and agricultural land than cotton. Also, their fibers are more durable, can be washed at lower temperatures, dry quickly and rarely need ironing. Although their production depends on oil feedstock and requires intensive energy, all can be recycled into new fibers.
Man-made fibers derived from natural polymers such as cellulose have been obtained for centuries by dissolving and regenerating biomass. The global production of man-made cellulosics (MMCs) more than doubled from 1990 to 2017 (Šajn 2019). In 2019, the annual output of man-made cellulose (MMC) was 7.1 million tons (about 6.4% of the total fiber production), while cotton and synthetic fibers contributed to 23.2% and 62.9% (52.2% polyester and 10.7% from others), respectively (Exchange 2020). In the EU market of textiles, only about 9% was wood fiber based (Šajn 2019), but cellulosic fibers are still absolutely necessary for some textile applications due to their unique absorbency and moisture management (Mendes et al. 2021). Man-made fibers include acetates, nitrates and ethers, but the most commonly used is viscose, which is also known as rayon. Recently, the textile industry has developed novel materials that are more sustainable, such as Lyocell (also known under the brand name of Tencel™), Bemberg™ (the brand name for cupro, made from the fuzz around the cotton seed, which is not normally used as fiber), Piñatex® (from waste leaves of pineapple plants), and Lyohemp® (from the bast fibers of hemp).
Inorganic man-made fibers are obtained from materials such as glass, metal, carbon or ceramics, and often used to reinforce plastics to form composites.
Based on recent statistics, around 73% of the world’s used textiles end up in landfills or incinerators, thus resulting in a huge loss of resources and economic value. In fact, clothing textile waste has been estimated to result in US$ 500 billion losses of value each year (de Oliveira et al. 2021). Globally, less than half of used clothes are collected for reuse or recycling when they are at the end of their life cycle, but only 1% is recycled into new clothes (Ellen Macarthur Foundation 2017). This is explained because in most cases, textile waste is a blend of natural and synthetic fibers, (particularly cotton/polyester mixtures) that makes fractionation and/or separation rather complex. Moreover, the lack of traceability and automatic technologies for sorting collected waste textiles, the need for pretreatments to remove colors during cleaning and discoloration of fibers from dyes and pigments, and the difficulty in separating finishing additives that are added to fibers in order to give them added properties are some of the common challenges that hinders the recyclability in post-consumer textiles (Kahoush and Kadi 2022). Clothes continue to be recycled mechanically, usually cut up and shredded, which means that resulting fibers have lower quality, affecting mechanical properties. Therefore, they are unsuitable for respinning and manufacturing new clothes. Although technologies for chemical recycling that produce virgin fibers of high quality are available for polyester and nylon, they are still economically unfeasible for cotton and blends (Šajn 2019).
In order to influence the change of textile recycling, the waste directive UE 2018/851 will require EU countries to collect waste textiles separately by 2025. This measure is expected to increase the fraction of collected post-consumer textiles and reduce the amount of clothes that are sent to landfills or being incinerated.
The transition to a circular economy will require textile industry to address the problems posed by fast fashion practices (i.e., over-production), adopt sustainable processes to transform raw material into textile fibers (i.e., fiber production), and invest in effective methods for recovering and valorizing waste materials by using them as a feedstock for other purposes (i.e., establish recycling initiatives). Since lignocellulose is the most abundant biodegradable polymer on Earth, adopting this natural material as a source for textile fibers can greatly help reduce the dependence on oil and allow sustainability concerns to be addressed.
This review provides an in-depth introduction to the potential of cellulose as a source of textile fibers. Thus, it discusses dissolving pulp properties and manufacturing processes, including upgrading processes from paper source. Also, it describes the uses and applications of dissolving pulps as textile regenerated cellulose fibers, highlighting the novel processes based on environmentally friendly technologies, and identifies needs for future research into effective textile waste recycling strategies for obtaining high value-added products.
Methodology
We searched the Web of Science, Scopus and Google Scholar databases. The keywords used were “dissolving pulp,” “upgrading dissolving pulp,” “textile fibers,” “hemicellulose removal,” “man-made fibers,” “regenerated cellulose,” “viscose,” “ionic liquid” and “cellulose dissolution.” Articles and reviews were screened and analyzed for results and relevant findings. As shown in Fig. 3, dissolving-grade pulp has been a topic of interest during last 10 years as demonstrated by different studies reported in the literature.
Biomass components
Lignocellulose is comprised of three polymers namely lignin [C9H10O3(OCH3)0.9−1.7]x, hemicellulose (C5H8O4)m and cellulose (C6H10O5)n, accompanied by minor amounts of other compounds such as proteins, ash, and pectin (Fig. 4) (Sjöström 1993; Fengel and Wegener 2011; Habibi 2014). The three components are covalently linked to form a complex and tridimensional network known as “lignin-carbohydrate complexes” (LCC). For this reason, lignocellulosic materials are considered as a lignocellulosic matrix rather than individual fractions.

Source: https://www.frontiersin.org/articles/10.3389/fenrg.2018.00141/full. (Baruah et al. 2018)
Structure of lignocellulosic biomass components. Creative Commons Attribution License (CC BY).
Lignocellulosic biomass consists of a large number of cells that can be imagined as hollow tubes with multilayered walls. The so-called compound middle lamella (CML) connects individual cells together. CML, which is amorphous and lignin rich, is the area between neighboring cells rather than a part of the cell wall. The true cell wall is an open network of microfibrils embedded in an amorphous material. It is subdivided into three separate layers: S1, S2, and S3 (Fengel 1971). Cellulose is the main component and is presented in S2 layer, whereas hemicellulose is found in the cell wall. Cellulose forms highly ordered elementary fibrils approximately 3 nm across, that in turn organize into bundles that form microfibrils with amorphous polysaccharides between them.
Cellulose
Nature produces about 1011–1012 tons of cellulose each year (Hon 1994; Rosenau et al. 2001; Habibi 2014; Heinze et al. 2018). In fact, this component can be obtained from different natural sources including wood, plants, algae and tunicates (marine animals), and can also be produced by bacteria.
From a chemical point of view, cellulose is a linear homopolymer composed of anhydro-β-D-glucopyranose units (AGU) that are linked together by (1-β-4) glycosidic bonds. Cellobiose, consisting of two AGUs molecules, is the repeating unit in the polymer (Sjöström 1993; Fengel and Wegener 2011). The number of AGU determines the length of cellulose chain, (i.e., its degree of polymerization, DP); however, the DP in cellulose is greatly dependent on the origin and the method of isolation.
Each AGU unit has two secondary hydroxyl groups at C2 and C3, and a primary hydroxyl group at C6. One end contains an alcoholic OH group at C4 (non-reducing end), while on the other end, C1 is part of an aldehyde group with reducing activity. These hydroxyl groups are responsible for forming both intra- and intermolecular hydrogen (H)-bonding network (Fig. 5).
Intramolecular (a) and Intermolecular (b) hydrogen bonds in cellulose (Lin and Dufresne 2014)
Hydrogen bonds are responsible for the structure of cellulose, and also for its insolubility in water and common organic solvents. The characteristic rigidity is via co-crystallization of multiple chains into parallel structures forming elementary fibrils that further organize themselves as microfibrils (Fig. 6) (Kontturi et al. 2006; Fengel and Wegener 2011; Gandini 2011; Moon et al. 2011). However, the fundamental reason behind cellulose almost non-dissolving properties remains controversial.

Source: https://www.nature.com/articles/s41598-020-66916-8. (Jakes et al. 2020)
Breakdown of softwood from the cellular to nanoscale. A Creative Commons Attribution license (CC BY).
The supramolecular model of cellulose is based on the organization of cellulose chains (Fig. 6). During its biosynthesis, cellulose chains aggregate into microfibrils that are micrometers long and 5–20 nm in cross- sectional width (Klemm et al. 2005). The fibrils contain both ordered and disordered components (Atalla and VanderHart 1999; Viëtor et al. 2002). Amorphous or less ordered regions are consequence of tilts and twists in microfibrils, hindering correct packing of cellulose. These “defects” create regions where cellulose is not well-packed and are thus more accessible to chemicals and reactants. Crystalline regions where cellulose is tightly packed are present between the amorphous zones where intermolecular hydrogen bonds are properly formed and stabilize the structure as a result. Variations in hydrogen-bonding networks and molecular orientation result in different cellulose polymorphs or allomorphs, depending on the source, extraction method and pretreatment used (Atalla and VanderHart 1999; Viëtor et al. 2002).
Crystalline cellulose exists in the polymorphs of cellulose I, II, III, IV (Ishikawa et al. 1997; O’Sullivan 1997; Moon et al. 2011). Cellulose I, which is the form found in nature, is produced by trees, tunicates, algae, plants and bacteria. This cellulose form consists of sheets stacked together by H-bonds, and Van der Waals interactions contribute significantly to the stiffness and specific structure of cellulose (Wada et al. 2010). Cellulose I occurs in two allomorphs, Iα and Iβ, with different parallel crystalline structures and different arrangement of cellulose molecules in the unit cell (Nishiyama et al. 2003). Cellulose I is thermodynamically metastable and can be converted to either cellulose II or III.
Cellulose II is the most stable form of technical relevance and can be produced by regeneration (solubilization and precipitation) or mercerization (with aqueous sodium hydroxide treatments) (Klemm et al. 2005). Mercerization typically occurs at NaOH concentrations above 8–9% (Sixta 2008) and the conversion is rapid. Under these concentrations, the most severe changes materialize in the crystallinity which depend not only on alkalinity but also on treatment time (Borysiak and Doczekalska 2008). During this conversion, cellulose II forms antiparallel chains. Thus, every second chain is of opposite polarity to the next and there is one more hydrogen bond per glucose residue than in cellulose I.
Cellulose III can be formed from Cellulose I or II through liquid ammonia or organic amine treatments, and subsequent thermal treatments lead to Cellulose IV. The crystalline regions of cellulose are interspersed with less ordered paracrystalline or amorphous areas, resulting from imperfect packing and interactions with other non-cellulosic polysaccharides. Those amorphous areas are found on the surface (Larsson et al. 1997), but also along cellulose microfibrils (Wada et al. 2004).
Hemicellulose
Hemicellulose, which is the second most abundant polysaccharide in plant biomass in mass terms after cellulose, forms an amorphous matrix with lignin in which cellulose microfibrils are embedded. The monosaccharides constituting hemicellulose include pentosans (d-xylose and l-arabinose), hexosans (d-glucose, d-galactose, l-galactose, d-mannose, l-rhamnose, l-fucose) and uronic acids (d-glucuronic acid, d-galacturonic acid). Generally, the units of main chain are linked together by β-(1–4) linkages, although some species combine β-(1–4) and β-(1–3) linkages (Sjöström and Westermark 1999). The composition and relative amount of hemicellulose in the cell wall vary depending on the type of wood and non-wood species. Hemicelluloses are associated with cellulose microfibrils by hydrogen bonding and to other cell wall constituents via covalent linkage (Fengel 1971; Sjöström 1993; Fengel and Wegener 2011). Hemicellulose contributes to the strength formation of cell wall in association with cellulose microfibrils and to bring flexibility to the structural assembly of cell wall components. The presence of hemicelluloses also inhibits the coalescence of cellulose microfibrils, thereby improving fibrillation of cellulose into nanosized fibrils. Hemicellulose possesses side groups on the chain molecules (in the amorphous form), making them easy to dissolve and degrade in acid and alkaline solutions (Olszewska 2013).
Lignin
Lignin, an aromatic heteropolymer, is composed of 4-phenylpropane structural units (guaiacylpropane, G; syringylpropane, S; and hydroxyphenylpropane, H) bound by ether and carbon–carbon bonds (Hammel 1997).
The composition of lignin depends on its botanical origin. Thus, softwood lignin has mainly G units, whereas hardwood lignin contains G and S units, and non-wood lignin H, G and S units, in different proportions. S units are known to be more reactive than G units, making lignin rich in S units easier to remove by pulp delignification (Del Río et al. 2001).
Lignin represents between 25 and 33% of dry softwood biomass and about 18–34% of hardwood biomass. It is produced by maturing cells and it is located between fibrous walls mainly in intercellular regions (middle lamella), creating a stiff and cohesive structure (Fengel and Wegener 2011). The covalent union between lignin, cellulose and hemicellulose means that lignin removal involves degradation of carbohydrates.
Extractives
In addition to the above-described major constituents, wood and non-wood materials also contain small quantities of extractives, between 2 and 10% depending on the particular wood species. Extractives, which are mainly resin and fatty acids and esters (Gullichsen et al. 1999), are partly soluble in water or organic solvents, but they can pose significant challenge in wood pulping. In fact, extractives released from fibers can form colloidal pitch and deposit in water circuits, leading to production problems (Gutiérrez et al. 2001).
Cellulose sources: wood and non-wood fibers
Wood and plant fibers differ in their cellulose content, with wood having generally 40–50% w/w of cellulose, and plant fibers are in the range of 30–75% w/w. Wood fibers have higher content of hemicellulose and lignin compared to plant fibers, mainly for lignin, which shows a content of about 30% w/w in wood fibers and only about 5% w/w in plant fibers. In addition, wood fibers exhibit lower cellulose crystallinity with typical values in the ranges of 55–70, whereas plant fibers have values in the range of 90–95% w/w (Madsen and Gamstedt 2013).
Wood fibers can be classified into softwood or hardwood fibers. Softwood is obtained from conifers and is long and resistant. These kinds of trees have a large proportion of fibers in wood, while other components are practically not present. Examples of softwoods would be trees such as pine, spruce or firs. Hardwood is obtained from homonym plants and, in evolutive terms, is newer organisms than conifers. Fibers are shorter than softwoods, are accompanied by other elements besides fibers, such as vessels, which play important roles in plant vital functions. Examples of hardwoods would be trees such as beech, eucalyptus or birch. Softwood hemicellulose consists of both pentosans and hexosans, while hardwood hemicellulose consists mainly of pentosans. Broadly, the pentosans content in softwoods is about 7–10%, and in hardwoods, it ranges from 19 to 25%, depending on wood species (Li et al. 2018).
Under the non-wood fiber source, a heterogeneous range of plants with widely differing characteristics is included. The main sources of non-woody raw materials are agricultural residues from monocotyledons, including cereal straw and bagasse, or plants grown specifically for the fiber, such as bamboo, reeds, and some other high-fiber content plants such as flax, hemp, kenaf, coir, jute, sisal, ramie or abaca (Marques et al. 2010). These non-woody plant-based fibers held a market share of approximately 5.8% of the total global fiber production volume in 2019, which corresponded to a global production volume of around 6.5 million tons (Exchange 2020).
Cotton is another sort of non-wood fiber that mainly supplies the textile industry. The cotton plant is an annual shrub, which grows in the subtropical and tropical regions of north and south of the Equator. The cotton flowers seed capsules consist of 30–40 oil-containing seeds, each capable of producing about 5,000 up to 20,000 single seed hairs (i.e., cotton fibers) (Heinze et al. 2018), and are basically comprised of lint and linters. The lint (staple cotton) is the long-fiber population, and linters are the short and thick-walled fibers of the fuzz (Heinze et al. 2018). Although the main purpose for growing cotton was and still is to obtain the staple cotton fiber (lint) for the textile industry, cotton linters as by-product are considered a valuable cellulose raw material (98%) and these high-quality fibers are mainly used in special applications such as the production of cellulose derivatives, regenerated cellulose, or the manufacture of high value-added papers (Sczostak 2009).
As can be seen in Fig. 7, OECD-FAO (2023) estimates that the cotton production will continue to increase by approximately 1.7%/yr up to 2032, but the consumption of other fibers is expected to grow at faster rate. Actually, the cotton consumption is forecast to grow at 1.98%/yr, which is slower than production projections.
Evolution and projections of world cotton production and consumption. Data from: Compare your country: http://www.compareyourcountry.org/agricultural-outlook/en/2/CT/all/default/all/CT Accessed: June 2023
Dissolving-grade pulp
Dissolving-grade pulp is a highly pure pulp with a high α-cellulose content (over 90%) and low levels of hemicellulose (< 4%), lignin and extractives.
Initially, it was thought that only cotton and softwoods were suitable for obtaining dissolving pulps, but broader range of hardwoods have also proved suitable and economically attractive for this purpose since the 1940s (Köpcke 2010). At present, about 85% of dissolving pulp is made from softwood, followed by hardwood, and lastly cotton linter with approximately 10% (Sixta 2008; Sixta et al. 2013). Among softwoods, the Pinus, Picea, Larix, Cedrus and Tsuga genus are the most employed in the production of dissolving pulp; and from hardwoods include Eucalyptus, Fagus, Betula, Populus, Acacia, Quercus and Acer genus (Mendes et al. 2021).
In recent years, investigations have been focused on the use of non-wood raw materials, such as bamboo (Ma et al. 2011; Batalha et al. 2012; Wu et al. 2014, 2017; Basit et al. 2018), bagasse (Andrade and Colodette 2014), jute (Nayeem et al. 2017; Sarkar et al. 2018), hemp (Zhang et al. 2006; Paulitz et al. 2017) and corn stalk (Behin and Zeyghami 2009) as potentially suitable raw materials for the production of dissolving pulps (Mendes et al. 2021). This is particularly of interest in Asian countries, since the production of dissolving pulp from bamboo has a market in China (Ma et al. 2011; Ji and Zhao 2015). However, the reactivity of bamboo-based dissolving pulp, obtained under similar pulping and bleaching conditions to wood-based dissolving pulps, is somewhat lower (Fu et al. 2012; Chen et al. 2016).
The worldwide production of dissolving pulp has increased since the beginning of the twenty-first century from a total output of 2.77 million tons in 2000 to 5.79 million in 2010, and reaching 8.86 million in 2020 (i.e., an increase by 50% relative to 2010) (FAO 2021) (Fig. 8). This growth can be attributed to various factors including (1) an increased consumption and production of regenerated textile fibers due to the interest in using cellulose as an alternative to petroleum-based products; (2) changes in fashion leading to low prices and shorter product lifespans; (3) a shortage of land for expansion resulting from competition with food crops; and (4) environmental concerns related to water-intensive cotton irrigation.
Evolution of dissolving wood pulp production. Data from FAO: https://www.fao.org/faostat/es/#data/FO. Accessed June 2023
Asia is currently the main producer, which contributes in 34.3%, followed by America (28.4%), Europe (24%) and Africa (13.3%) (FAO 2021).
Dissolving pulp can be chemically modified to obtain cellulose-based man-made materials, such as regenerated fibers or regenerated films (viscose, lyocell, cellophane), cellulose esters (acetates, nitrates, propionates, butyrates), cellulose ethers (carboxy methyl cellulose, methyl cellulose, ethyl cellulose, hydroxy ethyl cellulose, cyanoethyl cellulose), and micro- and nanocellulose (Sixta 2008; Miao et al. 2014; Wang et al. 2014; Chen et al. 2016; Stepan et al. 2016) (Fig. 9). Although the end-uses are very extensive, about 70% of the global dissolving pulp production is used for commodity applications, specifically viscose (rayon fibers) for the textile industry, while the rest of the production is used for manufacturing cellulose derivatives (Kumar and Christopher 2017; Yang et al. 2019).

Adapted from Woodings (2001a) and Sayyed et al. (2019)
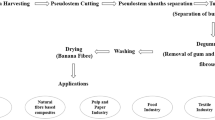
Schema of Dissolving Pulp applications.
Properties of dissolving-grade pulp
The most relevant quality-related characteristics of dissolving pulps are its α-cellulose content, alkali solubility, degree of polymerization, molecular weight distribution (MWD), and reactivity (Chen et al. 2016; Mendes et al. 2021):
α-cellulose content
Dissolving pulp is required to have a high purity, with α-cellulose content above 90%. Specifically, α-cellulose refers to the residual portion which is resistant to 17.5% NaOH (Tappi T-203cm-09). This α-cellulose has a high molecular weight. In general, hemicellulose is soluble in 18% NaOH (S18), whereas low-molecular weight cellulose (degraded cellulose) and hemicellulose are dissolved in 10% NaOH (S10) (Tappi T-235cm-00).
The hemicellulose content of DGP should ideally remain below 4%. For instance, a typical rayon grade dissolving pulp has a purity as α-cellulose of 92–94% in acid sulfite (AS) pulps and may reach up to 94–96% in pre-hydrolysis kraft process (PHK) hardwood pulps (Sixta 2008; Li et al. 2015b; Chen et al. 2016; Yang et al. 2019). Cellulose acetate must contain low residual hemicellulose since as amounts as low as 2.8% can severely affect acetate filterability (Zhao et al. 2017).
According to Yang et al. (2019), dissolving pulp should contain as little hemicellulose as possible because (1) the presence of remaining hemicellulose on fiber surface reduces its area and as a result the accessibility of cellulose to reagents; (2) hemicellulose is amorphous and has greater sensitivity to alkaline solution, which increases alkali viscosity and then the viscous alkali solution has difficulty in diffusing into cellulose microfibrils to react with cellulose, forming less homogeneous alkali celluloses; (3) it contains a large number of short chains and reducing ends that consume part of the oxygen, extending the pre-aging time required during the viscose process; (4) during the viscose process, carbon disulfide can react faster with amorphous hemicellulose than with cellulose, thus causing incomplete xanthation of cellulose and obtaining a poor final dissolution of cellulose xanthate (Christov and Prior 1993; Ibarra et al. 2010a); (5) hemicellulose can decrease the mechanical strength of the end-products (Strunk et al. 2012a); and (6) hemicelluloses (particularly xylose) also cause discoloration of viscose products (Sixta 2008).
Degree of polymerization (DP) and molecular weight distribution (MWD)
In most situations, pulps with lower degree of polymerization values exhibit higher cellulose dissolution (Wang et al. 2006; Evangelina Vallejos et al. 2022). A uniform MWD of the dissolving pulp is desirable in order to ensure homogenous reactions during the viscose process and provides improved mechanical properties of the final viscose fibers (Yang et al. 2019). The polydispersity index (PDI) is a parameter that determines the width of the MWD and is expressed by the ratio of the weight-averaged molecular weight and the number-averaged molecular weight of each sample (DPw/DPn). PDI is normally larger for AS than it is for PHK dissolving-grade pulps (Strunk et al. 2011; Testova et al. 2014; Duan et al. 2015b; Chen et al. 2016). This explains the lower yield of viscose fibers from AS than PHK due to the loss of low-molecular weight cellulose fraction in the dope preparation.
In general, the viscosity of dissolving pulp after cooking is about 600–800 mL/g, but the desirable level falls in the range of 400–600 mL/g. For viscose production, a moderate reduction in viscosity is a pre-requisite for better processability and end-product quality (Henriksson et al. 2005). Although the viscosity will further decrease to 200–250 mL/g during the downstream viscose process (Strunk et al. 2012b), very low viscosity can complicate filtration and reduce the physical strength of the final products (Chen et al. 2016). Therefore, it is essential to control DGP viscosity.
Reactivity and accessibility
The most complex challenge with cellulose polymer is achieving its dissolution because the highly crystalline and packed regions make it difficult.
As mentioned earlier, dissolving pulp is mainly used for the production of regenerated cellulose. In the mercerization of dissolving pulp, the crystalline region of cellulose must be accessible to the aqueous alkali in order to achieve the substitution of CS2 reactant in the following step (Fischer et al. 2009; Köpcke 2010). The degree of conversion and its reaction rate depend strongly on the availability of the hydroxyl groups to react with CS2 under predefined alkaline conditions (Fock 1959; Pönni 2014; Pönni et al. 2014; Tian et al. 2014).
In fact, reactivity relates to the accessibility of OH groups in the pulp and is influenced by a number of complex factors (Sixta 2008; Strunk et al. 2011; Li et al. 2018; Mendes et al. 2021). The fiber-related factors include fiber morphology, such as fiber length, width and cell wall thickness. For the microfibril or cell wall structure level, the influential factors comprise the microfibril characteristics (e.g., their angles and orientations), the pore structure of cell wall (pore volume, pore diameter, and pore distribution). Finally, the molecular factors include the chemical composition of raw material (species) and their distribution in the fiber/microfibril structures, the molecular weight and its distribution, the cellulose structure, the crystallinity and the ratio of cellulose I and cellulose II, and the contents and distribution of functional groups—which depends on the specific pulping method used (Henriksson et al. 2005; Sixta 2008; Duan et al. 2015a; Li et al. 2018). For example, reactivity decreases with increasing the degree of crystallinity, the dimensions of the crystallites and the fibril aggregates (Engström et al. 2006; Strunk et al. 2011; Ferreira et al. 2020). Therefore, increasing cellulose accessibility requires pores to be opened up, and fibril aggregates and highly ordered regions to be altered (Krässig 1993). The greater the reactivity is, the lower will be the consumption of reagents (CS2 and NaOH) during cellulose xanthogenation and the easier its dissolution in the aqueous alkaline solution (the so-called dope) (Ibarra et al. 2010a; Gondhalekar et al. 2019; Arce et al. 2020). Thus, high reactivity of dissolving pulps avoids many operational problems during the production of viscose (Chen et al. 2016).
The accessibility and reactivity are usually affected by microfibril coalescence (i.e., aggregation) during processing of the cellulosic material. The most widely accepted explanation for this phenomenon is the formation of exceptionally strong hydrogen bonds between the adjacent cellulose microfibrils (Newman 2004; Pönni 2014). Hydrogen bond formation is considered to occur mainly in the amorphous regions of cellulose. However, cross-linking between crystalline cellulose domains in adjacent cellulose microfibrils is considered another possible mechanism for the irreversible hydrogen bonding. Another approach to cellulose microfibril coalescence is the formation of lactone bridges (i.e., bonds between hydroxyl and carboxyl groups) (Fernandes Diniz et al. 2004; Pönni 2014).
The term hornification describes the physicochemical changes in chemical pulp fibers during drying (Fernandes Diniz et al. 2004). The removal of water from the cell walls of chemical pulp fibers causes almost all of the pores to collapse and lead to fibril aggregation, affecting negatively the reactivity of dissolving pulp. Coalescence in cellulose microfibrils can be induced by a number of factors and treatments. One is removal of lignin and hemicellulose from cell walls during chemical pulping. Dissolution of these components causes the formation of large pores that collapse when water is removed, thereby resulting in massive hornification (Maloney and Paulapuro 1999). The presence of hemicellulose has shown to avoid the hornification (Oksanen et al. 1997). Rebuzzi and co-authors (Rebuzzi and Evtuguin 2005) found that the presence of 4-O-methylglucuronoxylan (GX) control the extent of fibrils aggregation in pulp and reduce pulp hornification as a result. However, since dissolving pulp is characterized by low amount of hemicellulose, its removal makes the pulp prone to hornification. The changes induced by hornification reduce swelling and alter the strength properties (Maloney and Paulapuro 1999, 2000). The loss of strength is due to the stiffening of the fibers, which decreases the fiber–fiber bonding area (Maloney and Paulapuro 1999).
Reactivity affects the processability of dissolving-grade pulps in the viscose process, which has traditionally been assessed through filterability in the Treiber test (Treiber et al. 1962). The test reproduces the steps followed to convert pulp into viscose dope and measures the filtering time (s). The shorter the time is, the better is the viscose filterability. However, measuring filterability is a labor-intensive, time-consuming process that requires special equipment and relatively large amounts of pulp, so it is difficult to conduct on a laboratory scale (Ferreira et al. 2020). The Chinese Technical and Engineering Association uses an alternative method called “Chinese filterability test” that excludes several intermediate dope preparation steps. This method is commonly used in China and widely accepted, as China is a major viscose producer (Chen et al. 2016).
Fock’s test is another method that evaluates the amount of pulp that is dissolved in the viscose-like solution; therefore, the results are strongly influenced by pulp viscosity and by the amount of hemicellulose that is alkali-soluble. It is reported that, in most situations, Fock Reactivity increases with decreasing intrinsic viscosity in AS and PHK dissolving pulps (Engström et al. 2006; Strunk et al. 2011; Testova et al. 2014). High values of Fock solubility indicate high pulp reactivity. Fock reactivity should be at least 50% and values above 60% allow high viscose production to be achieved (Mendes et al. 2021). Importantly, Fock Reactivity (FR) and Chinese Filterability (CF) show a relationship to each other (Duan et al. 2015b).
The AS pulp is more accessible than the PHK pulp, supporting the conclusion that the former exhibits higher reactivity than the latter. Interestingly, the reactivity of hardwood PHK pulp was lower than that of softwood PHK pulp, even if the raw materials are processed identically (Ibarra et al. 2010a; Strunk et al. 2011). Bamboo dissolving pulp exhibits lower reactivity in comparison to wood dissolving pulps. The chemical composition of bamboo (specifically, its cellulose, hemicellulose and lignin contents) is similar to that of some hardwood and softwood species (Fu et al. 2012; Batalha et al. 2012); however, its fibers contain more vessels and multiple layers, and its secondary cell walls are more complex, all of which results in a lower reactivity (Fu et al. 2012; Chen et al. 2016). Wu et al. (2014) improved reactivity of bamboo dissolving pulp with a beating post-treatment. The beating increased the number of available hydroxyl groups, and the accessibility of chemicals to cellulose as a result.
Production of dissolving-grade pulp
Cotton is the only natural cellulose source that meets the characteristics of dissolving pulp: high α-cellulose content (over 90%), and low contents in hemicelluloses (< 4%), lignin and extractives. Cotton linters as extracted from seeds additionally contain other compounds that are not desired. Therefore, they require mechanical and chemical purification stages including (1) cleaning to eliminate physical impurities; (2) alkaline digestion in caustic soda in order to dissolve pectins, proteins and fats, while adjusting the DP; (3) bleaching (optional); and (4) finishing and drying if required for the intended end use. Moreover, issues related to the large land area required for farming and significant water consumption for irrigation result in high economic costs. Further, the so-called cotton gap has promoted the utilization of dissolving-grade fibers obtained from wood.
Dissolving pulp from wood source is produced by the acid sulfite process or pre-hydrolysis kraft process. Formerly, the AS process was the dominant process covering approximately 65% of the total dissolving pulp production and the PHK process for about 25% (Sixta 2008). By 2014, however, the AS process accounted for about 42% and the PHK process for about 56% of the global dissolving pulp production (Chen et al. 2016), a share that remains today. Importantly, dissolving pulp is more expensive to obtain than paper-grade pulp in terms of chemical consumption, production rate, inventories and storage space. It requires more extensive pulping and bleaching (Hillman 2006; Yang et al. 2019), and dissolving pulp yields are lower (30–38%) compared to kraft paper pulp (46–48%) (Mateos-Espejel et al. 2013; Kallio 2021).
The acid sulfite cooking is carried out using a strongly acidic cooking liquor (pH 1.5–2.0), containing an aqueous solution of sulfur dioxide and calcium, sodium magnesium or ammonium bisulfite. The cooking temperature is kept within a range of 125–145 °C and the cooking time is up to 7 h (Mboowa 2021). This process breaks down wood by dissolving lignin and cleaving the lignin bonds through the formation of sulfonate groups. Hemicellulose is hydrolyzed into soluble sugars, but cellulose remains unaffected. Therefore, hemicellulose, lignin and other minor components are removed from wood chips in the same process step and all components remain dissolved in the spent sulfite liquor (SSL), which can be then converted to high value-added products such as lignosulfonates, vanillin, xylitol, and ethanol (Lawford and Rousseau 1993; Magdzinski 2006) or burned for energy production. The brown stock is submitted to further purification steps, such as hot alkali extraction or various bleaching steps, to reach high-purity dissolving pulp (Chen et al. 2016).
The PHK process combines an acidic pretreatment (pre-hydrolysis) to extract hemicelluloses from the wood chips and hydrolyze them to soluble sugars, followed by kraft cooking under alkaline conditions in order to remove most of the lignin (Sixta 2008). Pre-hydrolysis stage removes most of hemicelluloses and a portion of lignin from the wood chips, due to auto-hydrolysis initiated by the generation of acetic acid. Also, a small amount of cellulose is hydrolyzed into short chains. The kraft cooking step removes most lignin present in the wood chips while hemicellulose and a fraction of cellulose are degraded and easily dissolved in the liquor. After cooking, the residual lignin is further removed from the pulp via multi-stage bleaching stages in order to achieve the desired purity of the dissolving pulp (Chen et al. 2016).
Besides differences in pulping process, differences in properties of both AS and PHK dissolving pulps have also been reported. For example, Sixta (2000) compared the properties of laboratory TCF bleached AS and PHK hardwood pulp, and found the former to have higher viscosity, higher content of low-molecular weight (MW) fraction, as well as broader molecular weight distribution. Additionally, PHK pulp exhibited higher α-cellulose content but lower reactivity. In the same line, Duan et al. (2015a) comparatively evaluated the properties of dissolving pulps obtained from the AS and PHK processes, in terms of purity, MWD, porosity, surface area, accessibility and reactivity. Overall, the PHK pulp had higher α-cellulose content, lower S10/S18 content, narrower MWD and lower polydispersity index (PDI) (3.8–4.5 vs 7.6–8.5) in comparison to AS pulps. These differences can be explained by different reaction mechanisms in the acid and alkaline pulping processes (Janzon et al. 2008; Duan et al. 2015a). Thus, in the AS pulping, acid hydrolysis reaction moves from the primary wall to the inner wall. The cleavage of glycosidic bonds under acidic conditions follows a non-uniform reaction and occurs randomly, resulting in a strong degree of polymerization across the cell wall, where the primary wall is largely destroyed (Sixta 2008; Duan et al. 2015a). In contrast, the PHK process contributes to a more uniform DP across cell walls thanks to the good swelling properties in the alkaline solution, thus resulting in a uniform impregnation and reaction, and keeping primary walls undamaged (Sixta 2000). Moreover, the broad MWD of the AS pulp relative to PHK pulp is associated with the higher amount of short-chain cellulose and higher alkali solubility of the AS pulp, even at a similar viscosity level (Strunk et al. 2011).
Regarding the wood raw materials that can be used for dissolving pulp, some softwood and hardwood species that are rich in extractives, such as tannins, polyphenols, pigments, resins and fats, are unsuitable for the AS process, because they can react with lignin to form condensed structures that will prevent the subsequent delignification (Sixta 1998). Moreover, taxifolin can decrease the stability of sulfite cooking liquor due to its conversion to thiosulfate (Chen et al. 2016).
Other methods such as the Organosolv process have been investigated for producing DGP but are not used on an industrial scale (Vila et al. 2004). Organosolv pulping involves hydrolyzing and delignifying wood with an organic solvent (mostly alcohol–water mixture), to increase the digestibility of cellulosic fraction. It has been investigated for several woody and non-woody biomasses and the process is effective for hemicelluloses/lignin degradation and cellulose crystallinity reduction (Geng et al. 2012; García et al. 2014). For economic and environmental reasons, the organic solvent must be recovered in most cases; additionally, the process is challenging, since a volatile and flammable solvent is used and requires to operate at high pressure (Baruah et al. 2018). Alternative solvents including glycols, phenols, esters, organic acids, acetone and amines able to work at similar pressure levels to those of conventional pulping processes (e.g., Kraft process) have been proposed as replacements (Rodríguez et al. 2018).
Purification treatments
After pulping process, some purification stages must be conducted in order to obtain a high-quality dissolving pulp. Such improvements on pulp quality are needed to meet the requirements of commonly obtained products such as viscose, acetates, cellulose nitrate or cellulose ether, among others (Ji and Zhao 2015; Arce et al. 2020). These purification treatments can be applied before or after bleaching treatments. The bleaching treatment is intended not only to enhance brightness, but also to increase purity, removing lignin and hemicelluloses, modifying the reactivity and adjusting the viscosity and molecular weight distribution of the cellulose (Liu et al. 2016) (Fig. 10). For the production of conventional dissolving pulp from PHK process, no additional purification steps are required for the removal of residual hemicelluloses. In contrast, the sulfite process typically includes hot caustic extraction (HCE) for purification of standard bleached dissolving pulp for viscose production (Sixta 2008; Syed et al. 2013; Kumar and Christopher 2017; Loureiro et al. 2021). Cold caustic extraction (CCE) is used in the production of dissolving pulp, when high purity is required, for example for cellulose acetate production (Sixta 2008). For instance, a typical viscose grade hardwood pulp may contain up to 5% of residual xylan, but the residual xylan content in acetate-grade pulps should not exceed 2% (Sixta 2008; Testova et al. 2014).
Conventional routes to obtain Dissolving Pulps (Schild and Sixta 2011)
Hot caustic extraction (HCE) as a post-treatment has been used for the production of dissolving pulps since the early 1940s (Hinck et al. 1985). HCE is an alkaline treatment that selectively removes short-chain hemicelluloses (particularly determined as S10 and S18 fractions). It uses low alkalinity (0.4–1.5% NaOH), high temperature (70–135 °C), and is based on chemical reactions such as alkaline peeling reactions of the polysaccharides and glycosidic bond cleavage. The efficiency of purification mainly depends on the sodium hydroxide concentration and temperature (Sixta 2008). In the case of HCE, fiber swelling is limited due to low alkali concentration, so hemicelluloses in the deep wall cannot be removed, although high cellulose yield loss is found due to elevated temperature conditions (Loureiro et al. 2021). HCE is largely used to obtain sulfite pulp and is not appropriate for kraft cooking since the carbohydrate degradation reactions follow the same mechanisms as alkaline cooking (i.e., oxidation of reducing-end groups) albeit under less severe conditions (Schild and Sixta 2011; Syed et al. 2013; Arnoul-Jarriault et al. 2015; Chen et al. 2016; Liu et al. 2016; Loureiro et al. 2021). HCE is not selective as CCE, and both, hemicelluloses and cellulose, are degraded by the alkaline peeling reaction mechanism. Generally, sodium hydroxide is entirely consumed by the neutralization of hydroxycarboxylic acids. Therefore, the alkaline lye cannot be recovered in the process, and hemicelluloses are not available for further valuable usage.
The CCE stage was first implemented on industrial scale during the early 1930s to produce high-quality dissolving pulps for nitration (Wallis and Wearne 1990). Cold caustic extraction involves fiber swelling and the extraction (dissolution) of short-chain carbohydrates, mainly hemicelluloses from the inner fiber to the bulk phase. CCE stage can be applied to dissolving pulps obtained from PHK and AS pulping processes. The typical process is conducted at 20–45 °C, and very high concentration of NaOH (8–10% in the liquor) is used, which requires an efficient recycling process of sodium hydroxide and additional washing, and heat-transfer capacity on an industrial scale (Loureiro et al. 2021). The cold caustic extraction becomes interesting because it provides both, high-purity dissolving pulps and high-quality hemi-caustic-lyes. Hemicelluloses in the CCE-lye are recovered in oligomeric and polymeric form as no further degradation reactions occur in the lye (Sixta 2008). The CCE stage is essentially a physical phenomenon and it is mainly influenced by the morphology of the fiber wall. Thus, the mechanism is explained by fiber swelling where a physical interaction between fiber and aqueous sodium hydroxide occurs, and the solubilization where the hemicelluloses diffuse through the pores of the fiber wall, from interior to exterior and then into the bulk solution. The solvent only penetrates into the accessible space between the microfibrils and the amorphous zones, but increased sodium hydroxide concentrations (above 8–9 wt%) can induce intracrystalline swelling through the cellulose fibrils. The conversion from cellulose I to cellulose II starts at 8–10 wt% alkali concentrations and is quantitative at 17–18 wt% (Sears et al. 1982; Klemm et al. 1998), slightly depending on the cellulosic raw material (Hirota et al. 2012). It is essential to keep the NaOH concentration low in order to ensure selective extraction of hemicellulose while avoiding the formation of cellulose II, which considerably affects cellulose accessibility—the latter has closer and denser structure due to its extensive intermolecule hydrogen bonding—and gives lower reactivity toward xanthation and acetylation (Sixta 2008). NaOH concentrations around 10 wt% at moderate temperature (20–40 °C) are usually sufficient to largely remove the hemicelluloses, which are mainly located in the intercrystalline areas. Arnoul-Jarriault et al. (2015) described the formation of cellulose II increased with the concentration of caustic soda and was correlated with the extraction of hemicellulose.
CCE stage consistently gives higher pulp yields than HCE. Also, CCE treated pulp has a narrow molecular weight distribution because the removal of hemicelluloses is based on physical swelling/solubilization mechanism without any degradation of the residual carbohydrates (Schild and Sixta 2011).
Figure 11 shows the mechanism of CCE and a combined mechanical refining and CCE treatment.
Schema of the combined mechanical refining and CCE concept for enhancing the hemicelluloses removal from a softwood sulfite pulp. a Original pulp fiber sample, b sample after conventional CCE (8–10% NaOH) treatment, c sample after mechanical refining and d sample after combined mechanical refining and CCE (4% NaOH. Reprinted from Publication Bioresource Technology, Vol. 192, Li Jianguo et al., Mechanical pretreatment improving hemicelluloses removal from cellulosic fibers during cold caustic extraction, 501–506, Copyright (2015), with permission from Elsevier (Li et al. 2015a)
Other purification treatments such as enzymatic removal of residual hemicellulose by xylanases and combinations of caustic extraction and xylanase treatments have been explored; all, however, have shown limited effectiveness on dissolving sulfite pulps as they remove xylan by 50% at most (Christov and Prior 1993; Gübitz et al. 1997). Christov and Prior (1993, 1996) confirmed that while dependent on treatment time and xylanase charge, enzyme hydrolysis of pulp xylan is limited mainly by the partial inaccessibility of the substrate. This may result from a number of factors such as enzyme size (Stone and Scallan 1968), fiber porosity (Wong et al. 1988), median pore size (Suurnäkki et al. 1997), and accessible surface area of pulp (Stone et al. 1969). Hemicellulose removal from dissolving pulp was improved when xylanase was complemented with another hydrolytic enzyme such as mannanase and endoglucanase (Gübitz et al. 1997). Also, combination of a mechanical refining and xylanase treatment have also been proposed to remove hemicelluloses from pre-hydrolysis kraft-based dissolving bamboo pulp, where fibrillation and disruption of fiber caused by mechanical forces enhanced the xylanase access to xylan (Zhao et al. 2017).
Quintana et al. (2015a, 2015b) studied the application of cold caustic extraction (CCE) stage and an endoglucanase treatment on a fully bleached softwood acid sulfite pulp and biobleached sulfite pulp in order to simultaneously improve cellulose reactivity and reduce hemicellulose content. They used two types of endoglucanases at two different doses, both independently and before or after CCE. Based on the results, it was shown that CCE reduced the hemicellulose content and this reduction was slightly improved with the combination of endoglucanase pre- or post-treatment. The best results in terms of Fock solubility were obtained with an endoglucanase treatment alone, whereas a cold caustic extraction by itself significantly diminished the values with respect to original pulp. This adverse effect was improved followed by endoglucanase treatment, but the results were still poorer than endoglucanase treatment alone. Friebel et al. (2019) also studied the optimization of caustic extraction of beech acid sulfite pulp using an oxygen-cold caustic extraction-ozone-peroxide (O-CE-Z-P) bleaching sequence, at a variable temperature and sodium hydroxide concentration in order to identify the conditions maximizing the xylan extraction yield. The bleaching sequence gave a dissolving pulp in outstanding purity with minimal cellulose degradation.
Upgrading dissolving pulp from paper or non-wood pulp
In China, cotton linter was the dominant raw material for the production of dissolving pulp accounting for 49.6% of the production, while 44.4% came from wood-derived dissolving pulp and the remaining was made from bamboo and other non-wood materials (Liu et al. 2016). However, in 2014, the production from cotton linters started to fall due to high prices and limited availability of this feedstock. Market settings in favor of wood-based dissolving pulps were the shortage of land for cotton expansion, in competition with land used for food production, and environmental concerns arising from the increased water and land requirements of cotton relative to woody biomass (Kumar and Christopher 2017).
The global output of cellulose fibers for the textile industry was 4.4 million tons in 2010, and rose to 7.3 million in 2022, only about 6.4% of the total fiber output, however. The growth rate of wood-based textile fibers relative to total fiber production was about 6.45%/year from 2010 to 2022 (Statista). If the demand for wood-based textile fibers continues at its current pace, then the annual production is expected to be 15 Mt higher in 2035 than it was in 2018, reaching a production of 23.5 Mt. On the other hand, if the demand grows at the same rate as GDP, the textile industry would consume 6-11Mt/year more of these pulps in 2035 than it did in 2018. Consequently, new investments in production facilities will be needed to cover the growing demand, because the demand growth ratio is higher than what has been projected for cotton and chemical textile fibers (Kallio 2021).
A variety of solutions including conversion of paper pulp mills to mills that can produce both paper and dissolving pulps depending on the needs of market have been proposed to increase the supply of dissolving pulps (Loureiro et al. 2021). However, this conversion still represents a major technical challenge, particularly for continuous operation (Mateos-Espejel et al. 2013; Arnoul-Jarriault et al. 2015). In China, about 14% of dissolving pulp was produced on a mill converted from paper grade in 2014 (Liu et al. 2016). Stora Enso (2019) converted a kraft pulp mill to dissolving pulp in Finland, and so did Risi (2013) in Canada and Sappi (2020) in the USA (Kallio 2021). While some mills have successfully transitioned, Greenfield mills would be also needed in order to meet the expected demand. In any case, the costs would be higher than converting existing paper mills (Kallio 2021).
One other way of obtaining highly purified pulp is by directly subjecting paper kraft pulps to pre- or post- purification stages. Conventional Kraft pulping offers the highest cellulose yield, but after cooking contains around 20% of hemicelluloses, which exceeds the standards for dissolving pulps (Sixta 2008). Extracting hemicellulose from paper kraft pulp enables to produce a broad range of products ranging from high-alpha dissolving pulps to high-yield paper-grade pulps; also, hemicellulose fraction may be processed as a value-added product. Therefore, the main challenges in upgrading paper pulp to dissolving-grade pulp are quantitative removal of hemicellulose, increasing the reactivity of the pulp and ensuring better control of its viscosity.
As can be seen from Fig. 12 and Table 1, upgrading paper pulp to dissolving-grade pulp has aroused great scientific interest as demonstrated by many studies reported in the literature.
Publications with the topic of (“dissolving-pulp” OR “dissolving-grade pulp” OR “upgrading dissolving pulp”), separating the once referred to upgrading dissolving pulp. Document types: Review article or article or meeting or others (no patents are included). Database: SCOPUS. Total documents: 936, and 298, respectively. Accessed May 2023
Hemicellulose removal
So far, CCE and HCE have only been applied as post-treatment to PHK process and sulfite process, respectively, in order to achieve very high pulp purity as requested for the production of acetate-grade pulp (Sixta 2008). As shown in Table 1, several researchers have applied CCE directly to paper pulp and the cellulose yield of a paper-grade pulp can be retained while substantial amounts of short-chain material are removed.
Experimental studies have confirmed successful extraction of xylan by using CCE to meet the specifications of viscose grade pulp, with a xylan content below 5%. However, glucomannan can only be extracted by 50%—compared to 90% xylan. This difference in behavior may be related to the presence of carboxyl groups in xylan molecules, which are responsible to improve the solubility in sodium hydroxide solution (Schild and Sixta 2011). For instance, Arnoul-Jarriault et al. (2015) studied two different hemicellulose removal strategies with the purpose to convert a softwood kraft paper pulp into a dissolving pulp. First, they examined the influence of CCE conditions such as NaOH load and temperature, and found both variables to have a substantial impact on xylan and glucomannan removal. Under the best experimental conditions (11% w/w NaOH and 85 °C), 80% of the xylan and 45% of the glucomannan in the pulp fibers were removed, which led to a final hemicellulose content of 5.4%. Although this value is on the higher side of common levels for dissolving pulp grades, it was observed that most of the cellulose was converted to cellulose II. They then used a combination of a hot acid treatment (A) at 150 °C and subsequent hot alkaline extraction (HCE) with 12% of NaOH on pulp at 110 °C. Under these conditions, a 63% extraction rate was achieved, that led to a residual hemicellulose content of approximately 6%. Importantly, this process did not contribute to the formation of cellulose II, but cellulose chains suffered a severe depolymerization and the hemicellulose content was in the upper range suitable for some dissolving pulp applications.
In contrast to xylan from pre-hydrolysis process, alkaline extracted xylan shows a high molecular weight, a high yield and purity (Schild and Sixta 2011). By contrast, residual resistant xylan presented a lower content of functional groups and a higher molecular weight which remained entrapped in the cellulose matrix. Using higher concentrations of alkali (> 10%) will convert cellulose I into cellulose II, which is not desired because the latter has lower reactivity. Therefore, additional purification steps such as xylanase and cellulase stages that selectively degrade residual xylan and depolymerize the cellulose macromolecules are required when aiming for high-purity cellulose with a low uniformity (Hutterer et al. 2017). Interestingly, Köpcke (2010) succeeded in removing hemicellulose while avoiding the formation of cellulose II with a xylanase pretreatment followed by mild alkali extraction (7% NaOH).
Xylanases (EC 3.2.1.8) are hydrolytic enzymes that catalyze the hydrolysis of xylans. They exhibit different behaviors in terms of endo- and exo-activity depending on the host organism as well as on the structure of the xylan substrate. The endoxylanase activity (endo-β-1,4 xylanases) randomly cleaves the xylan backbone, releasing oligomeric xylan substrates. On the other hand, the exo-activity (β-xylanases) results in the release of xylose monomers from reducing ends of xylooligomers in addition to xylan. Additionally, the removal of co-crystallized xylan, which acts as a physical barrier to the flow of reagents, aids the penetration of subsequent bleaching agents (Hutterer et al. 2017).
Köpcke et al. (2008) and Gehmayr and Sixta (2010) demonstrated the effectiveness of xylanase treatment followed by an alkali extraction and an endoglucanase stage at the end of the bleaching sequence to convert birch and eucalypt kraft paper pulps into high-quality dissolving pulps with hemicellulose content of 2–4% and 65–70% reactivity—which are comparable to the values for commercial sulfite dissolving pulp used in the viscose process. Beltramino et al. (2015) applied xylanase to modify sisal fibers to produce fibers with high cellulose content. The xylanase enzyme seemed to have found a limit in degrading xylans in sisal fibers, reaching a final content approximately 12% (initial 16%). To address this, a posterior alkaline extraction (4% w/v NaOH) was proposed, reaching a final content of 8.1%. Importantly, the combined treatment resulted in higher xylan removal than alkaline extraction alone.
Kaur et al. (2016) used xylanase to treat mixed hardwood kraft wood, leading to the solubilization of 29% of all pentosans present in the starting material (Yang et al. 2019). The efficiency of the xylanase treatment can be further enhanced by combining it with the mechanical refining process, which helps to increase the accessibility of xylan inside the fiber wall. While a single xylanase treatment may have limited effectiveness in removing hemicellulose, many studies in the literature (Köpcke 2010; Ibarra et al. 2010b; Gehmayr and Sixta 2011) support the application of xylanase as a pretreatment stage to enhance the hemicellulose removal in the subsequent alkali extraction stages or other purification treatments.
Loureiro et al. (2021) upgraded oxygen-delignified (ODL) paper-grade eucalyptus kraft pulp into dissolving pulp, without the use of a pre-hydrolysis step before kraft pulping. Various xylanases were used in combination with lytic polysaccharide monooxygenases (LPMO), a relatively recently discovered class of enzymes, to increase pulp purity. Purification was combined with hot and cold caustic extraction. The purification process was complemented with hot and cold caustic extraction. The bleaching sequence and the purification stages provided dissolving pulp that met the specifications for the manufacture of viscose: 2.8% xylan; viscosity of 8.5 cP; 92.4% ISO brightness; and 70% Fock’s reactivity.
An alternative purification method uses solvent systems containing metal complexes that have been found to selectively dissolve hemicellulose (Roselli et al. 2014). Nitren (tris(2-aminoethyl)amine nickel complex) dissolves xylan selectively in a simple manner: the pulp is extracted at 30 °C for 1 h, followed by filtration of the dissolved hemicelluloses. It can dissolve both xylan and cellulose, but the complexation of xylan is more favored than cellulose, as xylan can be solubilized at lower nitren concentrations (Burger et al. 1995; Saalwächter et al. 2000; Janzon et al. 2008). Puls et al. (2006) demonstrated the selective removal of xylans from hardwood paper pulps by nitren-extraction. These pulps feature a high purity level, high molar masses, a narrow molar mass distribution and the standards contents of carbonyl and carboxyl groups, while maintaining the cellulose I structure (Janzon et al. 2008). The extraction of hardwood pulps with 5–7% nitren solution results in a cellulose purity level of 93.6–95.9%, which is suitable for high-quality applications of dissolving pulps. Nevertheless, the production and recovery of nitren present some difficulties due to carcinogenic properties of many nickel compounds and allergic reactions caused by Ni itself. Another drawback is that nickel can easily contaminate the extracted pulp. Washing with lactic acid in two cycles with a total washing time of 60 min was necessary to reach a nickel concentration of 12 ppm in the final eucalypt pulp as reported by (Janzon et al. 2006; Sixta et al. 2013). Another cellulose solvent, Cuen (copper ethylenediamine complex), can also be used for purification of dissolving pulps (Burger et al. 1995; Puls et al. 2006). However, cuen is less selective in xylan removal as it also dissolves some of the cellulose, which affects the desired pulp purity (Puls et al. 2006). Most of the reported methods seem to be capable of converting paper-grade pulp to dissolving pulp, with various degree of success, but their impact on cellulose accessibility and reactivity requires detailed examination.
Ionic liquids (ILs) salts with an imidazolium derived cationic moiety have also proved good dissolution properties toward lignocellulosic biomass or even complex biopolymer matrices (King et al. 2009; Sun et al. 2009; Hummel et al. 2010; Mäki-Arvela et al. 2010; Brandt et al. 2013; Roselli et al. 2014). However, the dissolution capacity of ILs strongly depends on the water content (Mazza et al. 2009; Pinkert et al. 2009; Zakrzewska et al. 2010; Hauru et al. 2012).
Roselli et al. (2014) studied the extraction of hemicelluloses from paper-grade birch pulp by means of a 1-ethyl-3-methylimidazolium acetate–water mixture (Froschauer et al. 2013; Roselli et al. 2013; Sixta et al. 2013). Based on the preliminary study, a novel method called IONCELL was proposed, where hemicelluloses are extracted with an ionic liquid–water mixture such as 1-ethyl-3-methylimidazolium dimethylphosphate-water ([emim][DMP]) mixture. Both fractions can be recovered without yield losses or polymer degradation. IONCELL allowed bleached Eucalyptus urograndis kraft pulp to be refined to high-purity acetate dissolving-grade pulp and the hemicellulose content was reduced from its initial 16.6% to 2.4 wt%, while persevering the cellulose I crystal form. The xylan content of the pulp was further reduced to 1.66 wt%, by adding an endoxylanase pretreatment to the process. IONCELL process is under development, as requires additional funding for the piloting phase, as well as for further upscaling and commercialization activities (IONCELL 2023).
Recently, organic/polar cosolvents can also be added into ionic liquids, forming so-called organic electrolyte solutions (OES) (Yang et al. 2021a). OES solutions have several advantages over ionic liquids, including lower viscosity, and tunable physicochemical properties (Xu et al. 2013; Andanson et al. 2014; Clough 2017; Yang et al. 2021a). Yang et al. (2021a) explored the removal of hemicellulose in paper-grade hardwood bleached kraft pulp for upgrading to dissolving pulp. They used OES consisting of the ionic liquid 1-ethyl-3-methylimidazolium acetate (EminAc) and a polar organic solvent c-valerolactone (GVL). The resulting dissolving pulp (solid residue) reached a high cellulose content close to 95%.
Deep eutectic solvents (DES) provide another green solvent to be used for the conversion of paper-grade pulp to dissolving grade (biomass processing) (Abbott et al. 2003). These solvents are usually composed of a quaternary ammonium, phosphonium or sulfonium cation or a metal chloride (Smith et al. 2014), and have properties similar to those ionic liquids. Chen et al. (2021) investigated the effect of process conditions of lactic acid/choline chloride (10:1) in treating eucalyptus wood chips to obtain high-quality dissolving pulps. Chips were directly subjected to a DES pretreatment, followed by kraft pulping and bleaching. Dissolving pulp by DES-KP pulping process had an α-cellulose of 94.02% and Fock reactivity of 98.94% versus 89.81% and 60.97%, respectively, for PHK pulp (Chen et al. 2021).
Improving the reactivity
Mechanical treatment is proposed to improve the accessibility of cellulose, by increasing the fiber pore size, pore volume, fiber swelling and specific surface area. Mechanical treatment causes changes in the fiber morphology, fibrillation or cutting the fibers, and disrupts the tightly packed cellulose structure, making it more readily available to reactants (Tian et al. 2014; Miao et al. 2015; Li et al. 2018; Zhou et al. 2018; Yang et al. 2019).
PFI, Hollander beating (i.e., valley beating) and ball milling are technologies used to refine and beat pulp, each based on different principle and having different effects on cellulose. Mechanical treatments can affect both the internal and the external structure of the cellulose fibers. External fibrillation occurs when fine hair-like strands fray away from the fiber surface, while internal fibrillation occurs when the cross-linking hydrogen bonds are broken creating new exposed fiber ends and pores that are expanded under the repeating mechanical actions. Newly generated fiber ends have additional accessible sites that would lead to an increased accessibility of cellulose fibers. The extent of external and internal fibrillation can be controlled by adjusting the refining/grinding conditions (Li et al. 2018). For example, PFI is reported to cause internal fibrillation and swelling, whereas the Hollander beater has a greater cutting action, generates fines and external fibrillation (Ngene et al. 2022).
Li et al. (2015a) described that a mechanical refining prior to CCE increased the total pore volume, the specific surface area and fiber swelling, measured as WRV of softwood sulfite pulp. All this had a positive effect on the removal of hemicelluloses in the subsequent CCE stage, which reached a similar hemicellulose removal degree to conventional CCE process, but lowered the NaOH concentration from 8 to 4%, and the Fock reactivity was higher than that at conventional CCE processed with 8% NaOH. Ngene et al. (2022) proposed a new approach involving one-pot simultaneous refining and cold caustic extraction treatment with 6% NaOH to remove hemicelluloses from hardwood paper pulp. Interestingly, such treatment let to achieve a xylan removal similar to performing a sequential treatment but at higher NaOH concentration (10%). In any case, excessive mechanical treatment should be avoided as it can decrease pulp viscosity, and thus decrease the strength of the resulting rayon products (Tian et al. 2014). Also, when pulps are dried for shipment, the new fiber ends that are generated through the fibrillation effect are induced to form inter- and intra-fibril/fiber hydrogen bonds and the benefit of mechanical treatments may disappear. One effective strategy is to combine mechanical refining with an enzymatic and/or a chemical treatment, so that the accessibility of the inner regions of cellulose fibers to enzymes and other chemicals can be increased, and more importantly, the increased reactivity can be preserved even after drying (Grönqvist et al. 2014; Miao et al. 2014; Chen et al. 2016). One major drawback, however, is that mechanical treatments are usually energy-intensive.
Another strategy of improving the accessibility of cellulose is by using cellulases, a type of enzymes that hydrolyze the 1,4-β-D-glycosidic linkage of the cellulose chains to give glucose oligosaccharides (Ibarra et al. 2010a; Duan et al. 2015b). There are three major groups of cellulases, namely endoglucanases (EC. 3.2.1.4), cellobiohydrolases or exoglucanases (EC. 3.2.1.91), and glucosidases (EC. 3.2.1.21). Endoglucanase cleaves bonds randomly in the amorphous sites of the cellulose, and as a result, new chain ends are created and a rapid reduction in the DP is observed. Usually, oligosaccharides of different size and glucose are released as subproducts. Cellobiohydrolases also called exoglucanases (CBH), attack reducing and non-reducing ends of the cellulose chains, generating cellobiose units mainly. CBH can also act on microcrystalline cellulose by means of a peeling mechanism and rapidly produce soluble sugars while slowly decreasing DP. Glucosidases act on cellobiose (two glucose units linked by a 1,4-β-D-glucosidic bond) to give glucose units (Lynd et al. 2002). The degree of enzymatic hydrolysis is believed to depend mainly on three variables, namely cellulose crystallinity, specific surface area and the degree of polymerization of the cellulose. Most cellulases consist of two domains. One is a catalytic domain, which is responsible for the hydrolysis of the cellulose chain, whereas the other is a cellulose-binding domain (CBD) that helps the enzyme bind to the cellulose chain and bring the catalytic domain close to the substrate (Ek et al. 2009).
In general, cellulases can open the fiber structure, increase the number of pores or voids and its specific surface area (Miao et al. 2014). Consequently, enzyme-treated pulp shows increased exposure of crystalline surface, swelling ability and reactivity. Additionally, cellulase preferentially attacks the cellulose II allomorph, which is also thought to play a role in the increased reactivity of enzyme-treated pulp. Some researchers have used pure cellulose, such as cotton and microcrystalline cellulose (MCC, Avicel) as substrates to investigate the underlying mechanism. They reported that initially the enzyme attacks the outer layer of the cellulose surface, where the constituent fibers were peeled along their length, layer by layer, like an ‘‘onion” (Wang et al. 2006; Penttilä et al. 2010). Le Moigne et al. (2010) investigated the peeling effect of enzyme mixtures on the structural changes and alkaline solubility of two different sulfite dissolving pulps and concluded that the increased alkaline solubility of the enzymatically peeled substrates at a short time (less than 10 min) were due to a digestion of the primary wall, and a destructive action on the inside of the fiber (a decreased DP and an increased swelling of fibers). Engström et al. (2006) studied the feasibility of improving the reactivity of dissolving pulp with monocomponent endoglucanase and found it to decrease the viscosity and to substantially increase the reactivity.
Reactivity of pulp is strongly dependent on the specific surface area that can be accessed by the substrates (Zhu et al. 2009). Mechanical treatment can increase the specific surface area of dissolving pulp due to fiber fibrillation and fines formation. As confirmed by Tian et al. (2014), however, a mechanical treatment increases the reactivity only slightly. Combining mechanical treatment as a pretreatment to assist cellulase treatment provides more reaction sites for cellulase, thus enhancing their efficiency—and hence the reactivity of DGP (Yang et al. 2019).
Controlling the viscosity
Controlling the viscosity during the viscose process is crucial because too high levels can adversely affect cellulose processability (Henriksson et al. 2005; Engström et al. 2006; Kvarnlöf et al. 2006). A pre-aging stage in the viscose process reduces the viscosity to around 200–300 mL/g. In the dissolving pulp production, viscosity control is often carried out in the bleaching process. Endoglucanases also offer the possibility of reducing the viscosity, shortening or even eliminating the pre-aging stage. These enzymes target the less ordered cellulose found between and on the surface of fibril aggregates, as well as located in shorter segments within the fibrils. Attacking these regions results in fibrils being cut, viscosity lowered and the reactivity increased as a consequence (Henriksson et al. 2005; Ibarra et al. 2010a).
According to Yang et al. (2019), the reactivity enhancement and viscosity control of dissolving pulp are closely related. Thus, cellulases react with the cellulose chain to reduce its DP and increase its specific surface area. As a result, changes in reactivity and viscosity may occur simultaneously and affect each other. Also, cellulases can be combined with other methods such as refining, additive assistance or fractionation to control viscosity. Optimizing the treatment conditions is the key point to obtain high-reactivity dissolving pulp with desired viscosity (Yang et al. 2019).
Table 1 summarizes the treatments found in the literature to convert kraft paper pulps to dissolving-grade pulps.
Regenerated cellulose fibers (RCF)
Regenerated cellulose fibers are obtained by dissolving cellulose chemically, but without changing the chemical structure (Jiang et al. 2020). Textile applications require long fibers. Since the length of wood pulp fibers is insufficiently long, they need to be processed using continuous spinning and regeneration. Regenerated cellulose fibers offer unique characteristics found in both synthetic and natural fibers. Thus, they exhibit uniform morphological, mechanical, and physical properties, typical of synthetic fibers, and the biodegradability, CO2 neutrality, and low density typical of natural fibers (Felgueiras et al. 2021).
The regeneration of cellulose can be achieved by derivatization or direct dissolution (non-derivatization). In the derivatizing process, cellulose is modified before dissolution, whereas in non-derivatizing process cellulose is directly dissolved in solvent without any prior modification. The derivatizing process includes viscose and cellulose acetate and the direct dissolution includes cuprammonium, lyocell, alkyl imidazolium ionic liquids as relevant examples (Sayyed et al. 2019).
During derivatization, the chemical structure of the native cellulose is modified by adding another reagent to replace hydroxyl groups with other functional groups, and an intermediate compound such as methylol cellulose, cellulose nitrite, cellulose acetate and cellulose xanthate is formed as a result. These intermediate compounds are subsequently dissolved and processed before being regenerated into fibers. However, very few derivatives other than cellulose acetate and viscose have been spun into fibers and commercialized (Sayyed et al. 2019). On the other hand, the technology of direct cellulose dissolution is straightforward and reduces the use of chemicals by ten times compared to the viscose process. Moreover, the solvent is easier to recycle as it does not give any by-products, making it a more environmentally friendly option (Felgueiras et al. 2021).
Derivatization
Viscose
The viscose process is an industrial process that involves complex steps. Firstly, cellulose is dissolved in strong sodium hydroxide solution in order to swell the fibers, and an alkali cellulose (Na-Cell) is formed. This step is known as mercerization and high concentration of sodium hydroxide is able to convert cellulose I to cellulose II. Today, industrial mercerization is commonly done in a slurry with 17–19 wt% aq. NaOH at 45–55 °C (Woodings 2001). The alkali cellulose is pressed to obtain a precise ratio of alkali to cellulose and then shredded to provide adequate surface area for uniform reaction in subsequent steps, excess soda being recovered and re-used. Then, cellulose is aged by means of oxidative reactions in the presence of air (stored at 40 °C for 4-5 h) with the purpose to reach a desired DP, the feasible limit for which is 200–400 (Cornell 1960). A high hemicellulose content and low fiber porosity makes the pre-aging treatment difficult. After mercerization, the pulp is treated with carbon disulfide (CS2) at low temperature and pressure to obtain sodium cellulose xanthate (i.e., sodium salt of cellulose dithiocarbonate). The xanthate product is still in the fiber state (consistency around 10%), which is dissolved in dilute sodium hydroxide solution to obtain a viscous orange solution called “viscose.” Finally, the viscose solution is extruded through a spinnerette with very small holes into a spin-bath consisting of sulfuric acid, sodium sulfate, zinc sulfate, surfactant and water at 45–55 °C, being cellulose regenerated by the liberation of xanthate groups from its backbone. Rayon, either in the form of continuous filament (yarns or tow) or cut into staple, is washed and chemically treated (desulfurized) to remove impurities before applying a finishing process and packing (LaNieve and Richard 2007). The viscose process has certain limitations such as the clogging of the viscose filters due to non-homogeneous dissolution of cellulose or high hemicellulose content. Therefore, a maximum cellulose dissolution is always desired, but using large amount of CS2 than usual is not good because CS2 and also the by-products generated in the reaction (H2S gas and other volatile thio-compounds) are hazardous chemicals that may cause a negative environmental impact (malodorous, highly volatile, and flammable) (Vigliani 1954; Nevell and Zeronian 1985). Hence, many attempts have been made to develop more environmentally friendly processes based on alternative solvents that can directly dissolve cellulose such as Lyocell, IONCELL, DES, Cuen, among others (Table 2).
The regenerated cellulose fiber produced via the viscose process is commonly known as viscose or rayon (Chen et al. 2006; Wang et al. 2016; Kumar and Christopher 2017). Viscose is the dominant man-made cellulose fiber (MMCF), with a total market share of approximately 79%. Rayon fibers are a polymorph of cellulose II and do not have an organized structure in fibrils, as native cellulose fibers have. As a result, rayon fiber has an absence of orientation and a lower degree of crystallinity than cellulose fibers (Mitchell 1949; Mendes et al. 2021). Also, they are hydrophilic, and their high water retention capacity is an advantage for wet processing as this requires fast liquid absorption in the end-product (Ramamoorthy et al. 2015). Viscose fibers are weaker than cotton fibers (especially when wet) and tend to shrink more easily (Ramamoorthy et al. 2015). In comparison to Lyocell fibers, viscose fibers exhibit lower crystallinity, tensile strength, Young`s modulus, and thermal stability (Ramamoorthy et al. 2015). Viscose fibers are the regenerated cellulose fibers most widely used by the textile industry to manufacture clothes, linings, furnishing fabrics and household fabrics (Ciechańska et al. 2009). Viscose dope can also be used to produce cellophane films (Wawro et al. 2014), tire cords (Ramamoorthy et al. 2015), synthetic sponges and highly absorbent hygienic materials (Ciechańska et al. 2009; Mendes et al. 2021). However, 85% of all viscose production is in the form of staple fibers (Henriksson et al. 2005; Chen 2015).
According to the Global and China Viscose Report (2019), the global output of viscose fiber reached approximately 5.8 million tons in 2018. China is the largest producer and consumer of these fibers, with a sales market share close to 65% and a revenue market share near 61% (2018), followed by India with the sales market share over 10% (2017). The worldwide market for viscose fiber was valued at 13 290 million USD in 2020 and is expected to reach 18 270 million USD by the end of 2026.
Modal
Modal fibers are obtained by subjecting beech wood to a modified viscose process (e.g., using an increased load of CS2 for dope preparation, spinning solutions with higher DP values or using a different spinning bath composition and with addition of modifiers, which allows greater molecular orientation of fibers) (Albrecht 1981; Schaumann 1996; Ciechańska et al. 2009; Shen et al. 2010; Wang et al. 2016; Roeder 2017; Mendes et al. 2021).
Modal fibers are commercialized by Lenzing AG under the brand name of TENCEL™ (TENCEL 2023). They have a higher wet modulus, breaking tenacity and strength (in both wet and conditioned state), a lower water retention capacity, and higher resistance toward alkalinity relative to conventional viscose fibers (Albrecht 1981; Llaudet 1990; Schaumann 1996). In addition, they are less prone to swelling and exhibit a higher degree of cellulose polymerization and a more developed fibrillary structure than viscose (Albrecht 1981; Ciechańska et al. 2009; Shen et al. 2010; Wang et al. 2016; Roeder 2017). However, when compared with lyocell fibers, modal fibers have lower crystallinity and thermal stability (Ramamoorthy et al. 2015) (Table 2).
Interestingly, modal fibers exhibit exceptional softness, with long-lasting quality even after repeated washing, in addition to being silky smooth and gentle to the skin. Because of their good mechanical and overall comfort properties, these types of fibers are widely used in the production of different woven fabrics, underwear, sportswear and knit materials (Llaudet 1990; Roeder 2017; Latif et al. 2019), as well as in blends compatible with most textile fibers—fabrics made entirely from modal fibers require heavy ironing because their long fibers tend to pile up.
Modal fibers accounted for around 2.8% of the total MMCF market in 2019, with an output around 0.2 million tons (Exchange 2020).
Cellulose acetate
Cellulose acetate fiber is produced by acetylation, a reaction where hydroxyl groups in cellulose react with an excess of acetic anhydride (typically 5–15 wt%) and acetic acid in the presence of sulfuric acid. The most common form of cellulose acetate fibers has one acetate group for each 2–2.5 hydroxyl groups. This cellulose diacetate is known as “secondary acetate,” or simply as ‘‘acetate’’ (Fischer et al. 2008). Prior to the acetylation, cellulose is subjected to adequate shredding and swelling. Initially, the acetic anhydride reacts with the moisture in the pretreated cellulose, giving acetic acid and a completely anhydrous reaction medium. During dissolution, cellulose begins to react with acetic anhydride, in the presence of sulfuric acid as a catalyst. The initial reaction occurs mainly in the amorphous regions of the cellulose, and sulfate linkages are formed. When acetylation is virtually complete, the reaction mixture is viscous and clear. Excess acetic anhydride is then stopped with aqueous acetic acid that helps to remove the residual sulfate linkages by exchange with acetyl groups, and the presence of water reduces chain degradation (LaNieve and Richard 2007). The final cellulose triacetate or cellulose acetate should contain few sulfate groups in order not to impair properties such as color. Cellulose acetate is then dissolved in acetone, and the cellulose dope solution is filtered to remove the slightest impurities and lastly extruded through a spinneret with hole diameters ranging from 30 to 50 lm (Ertas and Uyar 2017). The yarns are produced and the solvent is evaporated in warm air in the subsequent step. This process is known as the “dry spinning” method.
Cellulose acetate accounted for around 13% of all MMCF, with a production of roughly 0.95 million tons, in 2019 (Exchange 2020). It is mainly used to obtain non-textiles such as cigarette filters (80% share), textiles and apparel (clothing, lining, felts, carpets) as the second-largest use (Sayyed et al. 2019). Because of its characteristics, softness and pleasant touch, and also good textile processing performance, cellulose acetate fiber has different textile applications. By contrast, it exhibits poor tensile strength, abrasion resistance and thermal stability, low durability and high tendency to gather static electricity. (Sayyed et al. 2019; Felgueiras et al. 2021). Therefore, for practical applications, cellulose acetate fibers are blended with other, strong enough fibers such as those of polyester. Quintana and co-authors synthesized acetylated cellulose films by subjecting biobleached dissolving pulp to a chemoenzymatic treatment (Quintana et al. 2018).
Other processes
Different alternative routes to produce man-made cellulose fibers have been investigated and realized, at least at the pilot plant scale (Protz et al. 2018). The CarbaCell® process, based on derivatization, is one of them (Fink et al. 2014). Rather than the reagent used in the viscose process, CS2, cellulose is reacted with urea (CO(NH2)2) to form an alkali-soluble cellulose carbamate (CC). The cellulose is dissolved in an alkali solution previously cooled below 0 °C, which can be then shaped very similar to conditions to those used for obtaining man-made cellulose fibers in the viscose process (Teng et al. 2018; Sjahro et al. 2021). Cellulose carbamate is a nonionic cellulose derivative rather than a polyelectrolyte like cellulose xanthate. As a result, the influence of precipitation conditions, especially electrolyte content like salt concentration as well as the nature of the salt, is much less pronounced in the CarbaCell® process than they are in the viscose process (Protz et al. 2018). The fact that both derivatives are alkali-soluble led Protz et al. (2018) to investigate the combination of both cellulose derivatives in solution, shaping the same into man-made cellulosic fibers and characterizing the resulting filaments. They obtained filaments very similar in textile physical properties to viscose filaments with a decreased carbon disulfide (CS2) input. Vallejos et al. (2022) assessed the possibility of obtaining regenerated cellulose products such as beads and films by cellulose carbamate solution, using eucalyptus sawdust as a raw material. The sawdust was processed by soda pulping and TCF bleaching sequence with the purpose to achieve the characteristics of dissolving pulps.
The greatest advantage of the carbamate process is that the cellulose carbamate is relatively stable at room temperature, so it can be stored with no loss of quality for over a year and transported without significant degradation (Klemm et al. 2005). Also, the carbamate dissolves much faster in caustic soda than xanthate and, unlike viscose, it requires no (post-)ripening (Fink et al. 2014). Other significant advantages of the carbamate process are that it is inexpensive and has low toxicity, requires low energy consumption and has a low environmental impact (Weißl et al. 2019; Sjahro et al. 2021).
Direct dissolution
Cuprammonium
The cuprammonium process is based on the dissolution of cellulose at a concentration of ca. 10% in a mixed solution of copper salts, ammonia with sodium hydroxide at low temperature, followed by precipitation of filaments in a coagulation bath (usually mineral acid solution, consisting of a dilute acid, an alcohol and a concentrated cresol solution). Cuprammonium cellulose is passed through small holes in a spinneret to regenerate cellulose in the form of multifilament yarns (Cook 2001; Kamide and Nishiyama 2001; Ciechańska et al. 2009; Sayyed et al. 2019). This process allows the use of either wood pulp with α-cellulose content ≥ 96% or cotton linters as raw material.
Cuprammonium fibers are extremely fine filaments, also referred to as “cupro fibers” or “ammonia silk” that exhibit higher tensile strength and lower tensile elongation than viscose fibers (Cook 2001; Kamide and Nishiyama 2001; Sayyed et al. 2019). The end uses of cupro fibers include a great variety of products such as chiffons, satins, and all manner of very sheer fabrics typically used to produce underwear, dress fabrics and linings (Cook 2001; Wang et al. 2016). Cupro fibers exhibit good tactile feeling to the human skin and can be easily combined with other fabrics. The commercial fabric is known by the trade name “Bemberg” and currently produced by Asahi Kasei Fibers Corporation (Wang et al. 2016). Despite all the valuable applications, this process is becoming less used due to environmental impact, mainly related to the hazardous chemicals used in the dissolution process (release copper in the waste stream), and also because of the high costs of the entire process due to the environmental regulation that must follow (recover and reuse water, chemicals with closed-loop) (Sayyed et al. 2019; Mendes et al. 2021) (Table 2). Cupro fibers have a market share of less than 1% of the total MMCF, and their only supplier, Asahi Kasei, manufactured 17 000 tons in 2019 (Exchange 2020).
Lyocell
Lyocell, which uses a polar, aqueous N-methylmorpholine oxide (NMMO) solvent to dissolve cellulose, appears to be the most promising process as a replacement for carbon disulfide process. The mechanism involves a direct dissolution of cellulose, so no formation of any intermediate cellulose derivative, such as alkalization and xanthation in the case of viscose process, is required. The excellent dissolving capacity of NMMO on cellulose is due to its strong N–O dipoles and basicity, making it an excellent hydrogen bond acceptor solvent. In the dissolution process, N–O bond, which has a stronger basicity than hydroxyl group, interact with the hydrogen atom on the hydroxyl group of cellulose and form new hydrogen bonds between NMMO and cellulose. Such bonds replace the intermolecular and intramolecular hydrogen bonds in cellulose in both crystalline and amorphous regions, thus disrupting the H-bond network of cellulose. This new and re-structural hydrogen bond network that is established, leads to cellulose dissolution (Zhang et al. 2018; Jiang et al. 2020).
The Lyocell process starts with thorough mixing of disintegrated dissolving-grade pulp with a pre-adjusted DP (either paper or dissolving pulp depending on the fiber use) in an aqueous NMMO solution (Fink et al. 2001). The typical conditions for cellulose dissolution are 50–60% NMMO, 20–30% water and 10-15% pulp, at 90–120 °C under reduced pressure. In the next step, water is evaporated in a film truder to obtain a desired dope solution with NMMO:water:cellulose ratio about 76:10:14 (Rosenau et al. 2001; Chavan and Patra 2004; Ciechańska et al. 2009; Hummel et al. 2015; Zhang et al. 2018; Jiang et al. 2020; Sayyed et al. 2020) that is degassed, filtered and spun through a spinneret into a coagulation bath consisting of a highly diluted aqueous NMMO solution to make filaments. Finally, the resulting staple fibers are washed, cut, finished and dried (Sixta et al. 2015). Temperature plays an important role in the process; thus, too low levels result in undissolved particles, whereas too high levels (≥ 120 °C) degrade cellulose (Sayyed et al. 2020; Mendes et al. 2021).
The main advantage of the NMMO solvent is the lack of toxicity, and it is biodegradable and has no harmful by-products (Chen et al. 2016), in contrast to the environmental issues related to carbon disulfide. In addition, it can be recovered by more than 99% for reuse from large-scale systems (Rosenau et al. 2001). Recycling the NMMO involves purification by filtration, adsorption, oxidation and ion exchange, and evaporation (Jiang et al. 2020).
Lyocell fibers known as Tencel® exhibit good mechanical properties (wet and dry strength, dry and wet tenacity, modulus of elasticity) and also good wearing properties (gloss and touch). However, they are more prone to wet fibrillation and costlier to produce than viscose fibers. Lyocell fibers exhibit a relatively high crystallinity, high orientation of the fibers, low lateral holds within fibers, quite large pore volume and a weak intrafibrillar hydrogen bond, which generates a fibrillation effect (Carrillo et al. 2004; Chavan and Patra 2004; Ciechańska et al. 2009; Ingildeev et al. 2013; Haule et al. 2016). They possess a smooth surface, quasi-round cross section as well as a homogeneous, dense fiber bundle structure (Hummel et al. 2015). These fibers also reveal some unique features such absorption, smoothness, contribute to breathability, resistance to bacterial growth and are gentle on skin (excellent touch) with long-lasting comfort (Woodings 1995; Ramamoorthy et al. 2015; Edgar and Zhang 2020; Jiang et al. 2020; Mendes et al. 2021).
The flexibility of the process in terms of the cellulosic raw materials is another important advantage. According to Rosenau et al. (2001), the process can in theory use paper-grade pulp, unbleached chemical pulp, cotton, rayon fibers or even waste paper, although problems with spinability may be encountered in some cases. As in the viscose process, certain substances can be added to the solution to tune fibers properties.
Lyocell process also has some drawbacks such as the side reactions in the cellulose-NMMO-water system during the dissolution of cellulose and the recovery or recycling of NMMO (viz., radical reactions, heterolytic deoxygenation, Polonowski type reaction and autocatalytic decomposition) (Rosenau et al. 2001). These side reactions and the resulting by-product can cause the degradation of cellulose, affect the final properties of the fibers, cause temporary or permanent discoloration of the spun fibers, a drop in product performance, a decomposition of NMMO and higher consumption of stabilizers. So, other alternative direct solvents for cellulose dissolution can be highly attractive for environmental and economic reasons (Rosenau et al. 2001; Hummel et al. 2015) (Table 2).
Lyocell is the third most used MMCF type after the viscose and cellulose acetate processes. It had a market share of around 4.3% and a production volume of 0.3 million tons in 2019, and is being estimated to grow faster than other MMCF (Exchange 2020).
Ionic liquids
Ionic liquids (ILs) are organic salts that exist as liquids at temperatures usually below 100 °C and consist of an organic cation and a smaller organic or inorganic anion (Wang et al. 2016; Cao et al. 2017). Several studies are described in the literature where ionic liquids (ILs) are used as solvent for cellulose dissolution (Swatloski et al. 2002; Derecskei and Derecskei-Kovacs 2006; Kosan et al. 2007; Cao et al. 2009; Wang et al. 2012; Roselli et al. 2014; Amini et al. 2021). They have aroused increasing attention on account of their chemical and thermal stability, non-flammability and low vapor pressure compared with traditional volatile organic solvents (Laus et al. 2005; Wang et al. 2012; Sixta et al. 2013; Roselli et al. 2014). Furthermore, they are almost completely recyclable (around 99.5%), and the amount of wastewater produced is much lower than that produced in the viscose process.
The physicochemical properties of IL can be adjusted by selecting an appropriate cation–anion combination. IL cations are typically alkyl pyridine, alkyl imidazolium, quaternary ammonium, quaternary phosphonium, and alkyl alkoxyammonium (Moniruzzaman and Goto 2011; Cao et al. 2017), whereas IL anions include chloride, bromide, or more complex structures such as hexafluorophosphate, trifluoromethyl sulfonate, bis(trifluoromethylsulfonyl)imide, and methylimidazolium chloride ([Amim]Cl). By far, the most successful families of ILs for cellulose dissolution are those based on the alkyl imidazolium cations. First generation ILs such as [Bmim]Cl and [Amim]Cl have proved effective in dissolving cellulose and especially suitable for cellulose spinning into fibers with good tensile properties. However, the use of halide-containing ILs is associated with several drawbacks. Thus, their typically high melting points afford high process temperatures, which cause severe cellulose degradation unless appropriate stabilizers are added. Furthermore, halides can easily corrode metal processing equipment (Hummel et al. 2015; Sixta et al. 2015), which has promoted their replacement with acetates and dialkylphosphates. These ILs demonstrate good dissolution characteristics, and it is possible to prepare cellulose dopes in concentration ranges that exhibit good spinnability using a dry–wet spinning process (Sayyed et al. 2019). The ability of Carboxylate-based IL are better solvents to dissolve cellulose and other polysaccharides under mild conditions than are chloride-based ILs, due to their strong hydrogen bond accepting capacity. They are less viscous than chloride-based IL and have melting points always below 0 °C. Spinning dopes with high cellulose concentrations (up to 20%) could be obtained using 1-ethyl-3-methylimidazolium acetate ([EMIM]OAc) without significant gel formation. The resulting fibers have viscoelastic properties comparable with those of the NMMO–Lyocell process. Cao et al. (2017) reviewed the latest studies on ILs for cellulose pretreatment, with special emphasis on imidazolium-based ones.
In the search for non-imidazolium-based ionic liquids, Michud et al. (2014) identified superbase-based ionic liquid 1,5-diazabicyclo[4.3.0]non-5-enium acetate [DBNH][OAc] as an excellent cellulose solvent. The studies conducted with the mentioned superbase-based ionic liquid revealed that the resultant cellulose solution had a high dissolution power and a low viscosity, with excellent spin stability resulting in outstanding fiber properties. The authors demonstrated the production of high-tenacity fibers, spun from a 17 wt% cellulose solution at a temperature below 80 °C, which were used to knit scarf and dress. IONCELL®, which uses the same dry–jet wet spinning method to obtain rayon fibers, has been deemed an effective alternative to the viscose and N-methylmorpholine N-oxide (NMMO)-based Lyocell processes. Their excellent properties make Ioncell-F fibers suitable as a reinforcing material for composites. However, solvent recovery and recycling still need to be improved (Michud et al. 2014) (Table 2).
Despite the promising technical performance, IL have aroused major environmental concerns as regards toxicity and biodegradability. In a recent work, Magina et al. (2021) evaluated the hazardous impact of ionic liquids and concluded that, based on available knowledge, there are still no reliable criteria for unequivocal attribution of toxicity and environmental impact credentials for ILs. Therefore, further investigations must be conducted to fully understand the mechanisms of dissolution of IL, and a more comprehensive database on the physicochemical properties of the ILs is required.
As far as environmental friendliness and economic viability is concerned, current technologies for complete removal of IL from cellulose involve extensive washing, and those for separating the water from the IL involve energy-intensive evaporation. Such a high energy demand should be reduced. In addition, the high cost of ILs has so far limited the potential development of using them in other industrial applications such as catalysis, electrochemistry organic synthesis and polymer chemistry among others (Wang et al. 2012; Amini et al. 2021).
Other solvents
Other non-derivatizing solvents for cellulose include LiCl/N,N-dimethylacetamide (LiCl/DMAc), aqueous sodium zincate solution (Biocelsol®), NaOH/thiourea/urea aqueous solution at a temperature below 0 °C and a cellulose concentration as high as 6–8% (CelluNova) (Kihlman et al. 2012), a tetra butyl ammonium fluoride/dimethyl sulfoxide system (DMSO/TBAF), metal complex solutions, and molten inorganic salt hydrates (Wang et al. 2016). These systems, however, are not commercially viable and are still under development due to some limitations. For example, the low volatility of LiCl/DMAc solvent causes cost and difficulties in recycling it. The NaOH/urea system requires further investigation to enhance the dissolving capacity and the stability of the cellulose solution. In fact, NaOH/thiourea system solvent cannot dissolve cellulose with a high degree of polymerization or materials with a cellulose concentration exceeding 10 wt%. Also, the dissolution process is energy-intensive and the resulting fibers have poor mechanical properties that are not suitable for textile applications (Alam and Christopher 2017).
Aqueous metal complex solutions are composed of transition metal ions and nitrous ligands the best known among which are cuprammonium hydroxide (Cuam) and cupriethylenediamine hydroxide (Cuen). The process generates heavy metal salts that are difficult to dispose of (Philipp 1993). In the Biocelsol process, the mechanical and enzymatical pretreatments open, activate and adjust de DP of dissolving pulps. Pretreated pulp is dissolved in cold aqueous sodium zincate (NaOH/ZnO) to obtain a mixture from which fibers are processed by conventional wet spinning. The main drawback of this process is the need to pretreat the pulp (mechanically for up to 5 h followed by a 2–3-h enzymatic hydrolysis with large amount of cellulolytic enzymes) (Vehviläinen et al. 2008; Grönqvist et al. 2015).
Grönqvist et al. (2015) identified more efficient conditions to pretreat cellulose pulp prior to dissolution. The alkaline solutions prepared from enzymatically treated pulp in an extruder had lower dope viscosities regarding the cellulose content than others prepared from state-of-the-art-treated pulp. This enabled the cellulose content in the dope to be increased to 7% (w/w) without increasing dope viscosity. This is a critical improvement for the following process steps in which the solution is regenerated into shaped products such as fibers. A more recent process uses periodate oxidation of pulp followed by cross-linking with chitosan to dissolve modified cellulose in dilute NaOH, and extrusion to obtain cellulosic fibers in the form of textile fibers (Alam and Christopher 2017). The process uses chitosan as a green cross-linker by taking advantage of the functionality of its amine groups to assist in forming covalent bonds. The produced fibers have a low content in aldehyde groups (∼2 mmol/g cellulose) and water retention values of 1.5–2.0 g/g fibers, comparable to those of cotton yarns. The process can be applied to hardwood and softwood pulps, and offers substantially higher yields than DGP as a raw material.
Another innovative, technically and economically feasible alternative is the NeoCel process, which comprises dissolution of pretreated dissolving pulp in a continuous-flow reactor in cold alkali, aided by Zn and additives, after which the spin dope is coagulated in a sodium carbonate solution. Bialik et al. (2020) conducted an investigation which included the design, optimization and modelling of a chemical recovery system for wet spinning of cellulose in sodium carbonate solutions (NeoCel). Based on the results, the system was technically feasible and reduced chemical makeup.
Yang et al. (2021b) developed an effective method for preparing wood textile fibers from natural wood. They treated natural wood with a deep eutectic solvent (DES), which exhibited a highly porous structure and excellent flexibility, so that it could be easily cut to separate the cellulose fiber bundles and then twisted to obtain textile fibers. Structural analyses and performance tests showed the satisfactory results in terms of weaving, tensile and elastic properties, and also of dyeability. Moreover, the wood textile fibers acquired some washing stability and antibacterial properties after a simple hydrophobic treatment.
Regenerated cellulose fibers, beyond their extensive use as textile fibers, exhibit other commercially available applications such as non-woven materials, sponges and wipes (Zhang et al. 2019; Santos et al. 2021). Other novel applications with great potential are regenerated cellulose aerogels (RCA), membranes or thin films for wastewater treatments, scaffolds for tissue engineering or as a reinforcement in composite materials (Wan et al. 2019; Armir et al. 2021; Maharjan et al. 2021; Medeiros Souza Kataoka et al. 2022).
Environmental impact of regenerated cellulose fibers
Life Cycle Assessment (LCA) is a comprehensive methodology utilized to assess and quantify the environmental impacts of products, processes or services throughout their entire life cycle, from raw material extraction to its final stages, such as disposal or recycling (Rosson and Byrne 2020).
Shen et al. (2010) assessed the environmental impact of man-made cellulose fibers by conducting an LCA on three types of fibers (namely viscose, Modal and Tencel produced by Lenzing AG, an Austrian company) and compared the results with those for other commodity fibers such as cotton, PET and PP. The study concluded that Tencel, Modal and Viscose (Austria) exhibited the lowest environmental impact among all studied fibers. Viscose (Asia) had a lower impact than cotton but comparable to that of PET; however, the material was less preferable than PP and other man-made cellulose fibers. Cotton was identified as the least preferred choice due to its high ecotoxicity impacts, eutrophication effects, and intensive water and land use. Angelstam et al. (2016) analyzed the full cradle-to-grave life cycle impacts of a T-shirt made from cotton or viscose. They focused on chemicals (pesticides, fertilizers), and water and land use, as these are the impact categories where cotton exhibits the heaviest burdens. The results showed that a viscose T-shirt had better environmental performance than one made from cotton.
Felgueiras et al. (2021) discussed the cradle-to-gate life cycle assessment of various cellulose fiber production methods by comparing data from different sources. It was concluded that Lyocell process was the most environmentally friendly process for cellulose fiber production available on the market. Furthermore, Schultz and Suresh (2017) compared the production of man-made cellulose fibers (viscose, lyocell and flax fibers included) in ten different scenarios, considering a comprehensive set of environmental impact categories. Fibers were assessed by using LCA in compliance with ISO 14044 and the draft LEO-S-002 standard. All relevant impacts associated with raw material extraction, dissolving pulp production and staple fiber production were examined—impacts from the use and end-of-life of MMCF were excluded, however. The authors concluded that the choice of MMCF raw material input significantly influenced the life cycle analysis of impacts. While no MMCF source was deemed environmentally preferable across all impact categories, Scenario 10 (Belgian Flax Production) performed well in most, followed by Scenario 5 (German Production from Recycled Pulp).
In conclusion, LCA provides a useful tool for evaluating the environmental impacts, providing insights into the most sustainable production methods and guiding the industry toward more eco-friendly choices.
Future prospects
At present, the majority of the raw materials used in global clothing production are based on virgin feedstock (over 97%), but particularly on synthetic fibers (63%) and cotton (26%) (Ellen Macarthur Foundation 2017; Haslinger et al. 2019). In Europe, 75% of textiles waste (4.3 million tons) are discarded annually due to the lack of viable recycling strategies. For that reason, the textile industry is seeking to extend the lifespan of textiles and promote their reuse in order to reduce the demand for virgin materials—and minimize the use of water, energy and chemicals along the production chain as a result. Promoting waste prevention requires both the textile industry to produce durable textile products that are suitable for long-lasting use, and consumers to be educated about the importance of preserving natural resources (Dahlbo et al. 2017). However, when it is no longer possible to reuse textile, recycling textile wastes into high value-added products holds great significance in terms of environmental protection and resource conservation, due to reduced reliance on fossil-based sources (Çay et al. 2020).
Zhou and Wang (2021) studied the feasibility of recycling waste cotton fabrics into biodegradable films for potential applications in food and agriculture. Selected T-shirts, bed sheets and jeans made of 100% cotton were assessed for solubility in different solvents such as sulfuric acid aqueous solution, NaOH/urea aqueous solution, and LiCl/DMAc. Similarly, Asaadi et al. (2016) demonstrated the conversion of cellulose-based waste material, such as cotton, cardboard, and paper, into new, high-tenacity Ioncell fibers by using [DBNH] [OAc] (Asaadi et al. 2016; Ma et al. 2016, 2018; Haslinger et al. 2019). Interestingly, [DBNH] [OAc] requires no stabilizing additives; also, it allows operation at 30 °C, which is a lower temperature than that required by conventionally spun solutions prepared from NMMO (Haslinger et al. 2018).
A substantial portion of all textiles made today contains blended fibers (interwoven cotton and polyester, mainly), and due to their heterogeneous nature, the recycling of these wastes is more difficult. For blended fabrics, recycling techniques are based on chemical treatments aimed at recovering the constituents (Ouchi et al. 2010; Palme et al. 2017). However, these treatments use strong acids and high temperatures that produce toxic effluents and consume large amounts of energy. Researchers have followed various approaches using different solvents to separate blended components (Haslinger et al. 2019; Paunonen et al. 2019; Navone et al. 2020; Yousef et al. 2020). For instance, cotton was isolated from blends with synthetic fibers by taking advantage of the selectivity of ionic liquids (De Silva et al. 2014; Lv et al. 2015; Haslinger et al. 2019; Xia et al. 2021), subjecting the cellulose to hydrothermal treatment (Hou et al., 2018), or degrading one of the components (e.g., PET) (Palme et al. 2017). Haslinger et al. (2019) successfully separated cotton and polyester by using [DBNH] [OAc], a superbase-based ionic liquid that affords the selective dissolution of the cellulose component. After filtration, the resulting cellulose solution can be directly used as a spinning dope for dry-jet wet spinning to obtain textile grade cellulose fibers (Hummel et al. 2015; Parviainen et al. 2015; Sixta et al. 2015; Stepan et al. 2016) comparable to commercial Lyocell fibers made from high-purity dissolving pulp. The treatment time in [DBNH] [OAc] was found to reduce the tensile properties (< 52%) and the molar mass distribution (< 51%) of PET under certain processing conditions. For example, Yousef et al. (2020) recovered cotton and polyester fibers from textile waste by removing dyes with a leaching solution of nitric acid at a concentration lower than 60%, followed by regeneration of spent acid with activated carbon. The process provided $1629/tons economic returns and a 96% recycling rate (Zhou and Wang 2021).
Other recycling approaches involve carbonizing textile waste into biochar (Çay et al. 2020), extracting cellulose nanocrystals (CNCs) (Huang et al. 2020; Vanzetto et al. 2021) producing aerogels (Zeng et al. 2019), using activated carbon or producing ethanol, biogas or cellulose acetate (Zhou and Wang 2021). Vanzetto et al. (2021) obtained spherical cellulose nanocrystals (SCNC) by oxidizing recovered textile waste with TEMPO. The oxidative treatment provided SCNC with a crystallinity index close to 80% and crystallite size less than 6 nm were obtained. Similarly, Huang et al. (2020) directly extracted CNCs from cotton textile waste and evaluated their application as reinforcing agents of soybean protein isolate films with potential application in food packaging.
The above-described examples testify that it is possible to obtain a high value-added product using waste from the textile industry, contributing significantly to circular economy and sustainable development in the textile industry.
Conclusion
The textile industry is one of the world’s major polluters. In order to achieve a more environmentally responsible textile sector, it will require using renewable raw materials, finding sustainable, less toxic solvents, developing more efficient and energy-saving manufacturing technologies and following more effective recycling approaches to reduce post-consumer waste. Certainly, the use of cellulose polymer, in the form of man-made cellulose fibers, can play an important role in driving this environmental transition.
In this review, we describe the cooking processes employed to obtain dissolving-grade pulps (DGP) and highlight those characteristics that make them the most suitable feedstock for the production of regenerated cellulose fibers and cellulose derivatives. The high purity of dissolving pulp is reached through purification stages, although alternative methodologies for upgrading paper pulp to dissolving pulp were also discussed. While enzymatic treatments are highly effective for purifying this type of pulp, the cost of enzyme is still considered as one of the biggest difficulties for industrial applications and it is absolutely necessary to increase the efficiency of enzymes.
The viscose process, which has been in use for over a century, is the well-established process for manufacturing regenerated cellulose fibers. Despite a natural and biodegradable polymer like cellulose is used as a raw material, the process continues to raise serious environmental concerns due to the use of hazardous chemicals. These concerns have promoted a search for alternative processes involving direct dissolution of cellulose in recyclable solvents, being the lyocell process the only direct dissolution process currently commercialized. Lyocell fibers possess appropriate properties for various textile applications and fulfill the environmental requirements. However, their high production costs and certain problems have led to the development of other promising options including advanced ionic liquids, deep eutectic solvents, alkali-based and concentrated inorganic salt solutions, none of which, however, is commercially available today.
In conclusion, more sustainable practices are still required, and a collaborative effort from researchers, industry players, and policymakers is essential to drive the adoption of sustainable practices by the textile industry.
References
Abbott AP, Capper G, Davies DL et al (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun 1:70–71
Alam MN, Christopher LP (2017) A novel, cost-effective and eco-friendly method for preparation of textile fibers from cellulosic pulps. Carbohydr Polym 173:253–258. https://doi.org/10.1016/j.carbpol.2017.06.005
Albrecht W (1981) Achievements and prospects in the field of man-made fibre modification. Fibre Chem 12:207–218. https://doi.org/10.1007/BF00548708
Amini E, Valls C, Roncero MB (2021) Ionic liquid-assisted bioconversion of lignocellulosic biomass for the development of value-added products. J Clean Prod 326:129275
Andanson J-M, Bordes E, Devémy J et al (2014) Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids. Green Chem 16:2528–2538
Andrade MF, Colodette JL (2014) Dissolving pulp production from sugar cane bagasse. Ind Crops Prod 52:58–64. https://doi.org/10.1016/j.indcrop.2013.09.041
Angelstam M, Artman A, Hanström J, Rodríguez DM, Uskali D (2016) Comparative LCA. Viscose vs cotton T-shirts. Division of Environmental Strategies Research, SEED, ABE, KTH
Arce C, Llano T, García P, Coz A (2020) Technical and environmental improvement of the bleaching sequence of dissolving pulp for fibre production. Cellulose 27:4079–4090. https://doi.org/10.1007/s10570-020-03065-1
Armir NAZ, Zulkifli A, Gunaseelan S et al (2021) Regenerated cellulose products for agricultural and their potential: a review. Polymers 13:3586. https://doi.org/10.3390/polym13203586
Arnoul-Jarriault B, Lachenal D, Chirat C, Heux L (2015) Upgrading softwood bleached kraft pulp to dissolving pulp by cold caustic treatment and acid-hot caustic treatment. Ind Crops Prod 65:565–571. https://doi.org/10.1016/j.indcrop.2014.09.051
Asaadi S, Hummel M, Hellsten S et al (2016) Renewable high-performance fibers from the chemical recycling of cotton waste utilizing an ionic liquid. Chemsuschem 9:3250–3258. https://doi.org/10.1002/cssc.201600680
Atalla RH, VanderHart DL (1999) The role of solid state [formula omitted] NMR spectroscopy in studies of the nature of native celluloses. Solid State Nucl Magn Reson 15:1–19. https://doi.org/10.1016/S0926-2040(99)00042-9
Baruah J, Nath BK, Sharma R et al (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6:1–19. https://doi.org/10.3389/fenrg.2018.00141
Basit A, Latif W, Baig SA, Afzal A (2018) The Mechanical and comfort properties of sustainable blended fabrics of bamboo with cotton and regenerated fibers. Cloth Text Res J 36:267–280. https://doi.org/10.1177/0887302X18782778
Batalha LAR, Colodette JL, Gomide JL et al (2012) Dissolving pulp production from bamboo. Bioresources 7:640–651
Behin J, Zeyghami M (2009) Dissolving pulp from corn stalk residue and waste water of Merox unit. Chem Eng J 152:26–35. https://doi.org/10.1016/j.cej.2009.03.024
Beltramino F, Valls C, Vidal T, Roncero MB (2015) Exploring the effects of treatments with carbohydrases to obtain a high-cellulose content pulp from a non-wood alkaline pulp. Carbohydr Polym 133:302–312. https://doi.org/10.1016/j.carbpol.2015.07.016
Bialik M, Jensen A, Kotilainen O et al (2020) Design, optimization and modelling of a chemical recovery system for wet spinning of cellulose in sodium carbonate solutions. Cellulose 27:8681–8693. https://doi.org/10.1007/s10570-020-03394-1
Borysiak S, Doczekalska B (2008) Research into the mercerization process of beech wood using the WAXS method. Fibres Text East Europe 6:101–103
Brandt A, Gräsvik J, Hallett JP, Welton T (2013) Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem 15:550–583
Burger J, Kettenbach G, Klüfers P (1995) Coordination equilibria in transition metal based cellulose solvents. In: Macromolecular symposia. Wiley, pp 113–126
Bywater N (2011) The global viscose fibre industry in the 21st century—the first 10 years. Lenzinger Berichte 89:22–29
Cao Y, Wu J, Zhang J et al (2009) Room temperature ionic liquids (RTILs): a new and versatile platform for cellulose processing and derivatization. Chem Eng J 147:13–21. https://doi.org/10.1016/j.cej.2008.11.011
Cao Y, Zhang R, Cheng T et al (2017) Imidazolium-based ionic liquids for cellulose pretreatment: recent progresses and future perspectives. Appl Microbiol Biotechnol 101:521–532. https://doi.org/10.1007/s00253-016-8057-8
Carrillo F, Colom X, Suñol JJ, Saurina J (2004) Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur Polym J 40:2229–2234. https://doi.org/10.1016/j.eurpolymj.2004.05.003
Çay A, Yanık J, Akduman Ç et al (2020) Application of textile waste derived biochars onto cotton fabric for improved performance and functional properties. J Clean Prod 251:119664. https://doi.org/10.1016/j.jclepro.2019.119664
Chavan RB, Patra AK (2004) Review article: development and processing of lyocell. Indian J Fibre Text Res 29:483–492
Chen X, Burger C, Fang D et al (2006) X-ray studies of regenerated cellulose fibers wet spun from cotton linter pulp in NaOH/thiourea aqueous solutions. Polymer 47:2839–2848. https://doi.org/10.1016/j.polymer.2006.02.044
Chen C, Duan C, Li J et al (2016) Cellulose (dissolving pulp) manufacturing processes and properties: a mini-review. Bioresources 11:5553–5564. https://doi.org/10.15376/biores.11.2
Chen Y, Shen K, He Z et al (2021) Deep eutectic solvent recycling to prepare high purity dissolving pulp. Cellulose 28:11503–11517. https://doi.org/10.1007/s10570-021-04188-9
Chen J (2015) Synthetic textile fibers: regenerated cellulose fibers. In: Textiles and fashion: materials, design and technology. Woodhead Publishing Limited, Cambridge, England, pp 79–95
Christov LPP, Prior BAA (1993) Xylan removal from dissolving pulp using enzymes of Aureobasidium pullulans. Biotechnol Lett 15:1269–1274. https://doi.org/10.1007/BF00130310
Christov LP, Prior BA (1996) Repeated treatments with Aureobasidium pullulans hemicellulases and alkali enhance biobleaching of sulphite pulps. Enzyme Microb Technol 18:244–250. https://doi.org/10.1016/0141-0229(95)00058-5
Ciechańska D, Wesołowska E, Wawro D (2009) An introduction to cellulosic fibres. In: Handbook of textile fibre structure. Elsevier, pp 3–61
Clough MT (2017) Organic electrolyte solutions as versatile media for the dissolution and regeneration of cellulose. Green Chem 19:4754–4768
Cook JG (2001) Handbook of textile fibres: man-made fibers. II. Woodhead Publishing Limited, Cambridge
Cornell RH (1960) The uniformity of substitution during the emulsion xanthation of cellulose and the solution properties of the corresponding diethylacetamide derivatives. The Institute of Paper Chemistry. Appleton, Wisconsin, Doctor's Dissertation
Dahlbo H, Aalto K, Eskelinen H, Salmenperä H (2017) Increasing textile circulation—consequences and requirements. Sustain Prod Consum 9:44–57. https://doi.org/10.1016/j.spc.2016.06.005
Del Río JC, Gutiérrez A, Romero J et al (2001) Identification of residual lignin markers in eucalypt kraft pulps by Py–GC/MS. J Anal Appl Pyrolysis 58–59:425–439. https://doi.org/10.1016/S0165-2370(00)00126-1
de Medeiros Souza Kataoka LF, Leão RM, Gontijo AB et al (2022) Regenerated cellulose films from jute fibers applied in conductive nanocomposites. Mater Today Commun 33:104645. https://doi.org/10.1016/J.MTCOMM.2022.104645
de Oliveira CRS, da Silva Júnior AH, Mulinari J, Immich APS (2021) Textile re-engineering: eco-responsible solutions for a more sustainable industry. Sustain Prod Consum 28:1232–1248. https://doi.org/10.1016/j.spc.2021.08.001
Derecskei B, Derecskei-Kovacs A (2006) Molecular dynamic studies of the compatibility of some cellulose derivatives with selected ionic liquids. Mol Simul 32:109–115. https://doi.org/10.1080/08927020600669627
De Silva R, Wang X, Byrne N (2014) Recycling textiles: the use of ionic liquids in the separation of cotton polyester blends. RSC Adv 4:29094–29098. https://doi.org/10.1039/c4ra04306e
Duan C, Li J, Ma X et al (2015a) Comparison of acid sulfite (AS)—and prehydrolysis kraft (PHK)-based dissolving pulps. Cellulose 22:4017–4026. https://doi.org/10.1007/s10570-015-0781-1
Duan C, Verma Kumar S, Jianguo L et al (2015b) Viscosity control and reactivity improvements of cellulose fibers by cellulase treatment. Cellulose 23:269–276. https://doi.org/10.1007/s10570-015-0822-9
Edgar KJ, Zhang H (2020) Antibacterial modification of Lyocell fiber: a review. Carbohydr Polym 250:116932. https://doi.org/10.1016/j.carbpol.2020.116932
Ek M, Gellerstedt G, Henriksson G (2009) Wood chemistry and wood biotechnology. De Gruyter, Berlin
Ellen Macarthur Foundation (2017) A new textiles economy: Redesigning fashion’s future. https://www.ellenmacarthurfoundation.org/publications/a-new-textiles-economy-redesigning-fashions-future. London
El Seoud OA, Kostag M, Jedvert K, Malek NI (2020) Cellulose regeneration and chemical recycling: closing the “Cellulose Gap” using environmentally benign solvents. Macromol Mater Eng 305:1–21. https://doi.org/10.1002/mame.201900832
Engström A-C, Ek M, Henriksson G (2006) Improved accessibility and reactivity of dissolving pulp for the viscose process: pretreatment with monocomponent edoglucanase. Biomacromolecules 7:2027–2031. https://doi.org/10.1021/bm0509725
Ertas Y, Uyar T (2017) Fabrication of cellulose acetate/polybenzoxazine cross-linked electrospun nanofibrous membrane for water treatment. Carbohydr Polym 177:378–387
European Environment Agency (2019) Textiles in Europe’s circular economy. Briefing no. 10/2019. https://www.eea.europa.eu/publications/textiles-in-europes-circular-economy
European Environment Agency (2022) Textiles and the environment: the role of design in Europes circular economy. 1–18
Evangelina Vallejos M, Viviana Olmos G, Claudia Taleb M et al (2022) Dissolving pulp from eucalyptus sawdust for regenerated cellulose products. Cellulose 29:4645–4659. https://doi.org/10.1007/s10570-022-04581-y
Exchange T (2020) Preferred Fiber & Materials Market Report 2020. 103
FAO (2021). https://www.fao.org/faostat/es/#data/FO. Accessed June 2023
Felgueiras C, Azoia NG, Gonçalves C et al (2021) Trends on the cellulose-based textiles: raw materials and technologies. Front Bioeng Biotechnol 9:1–20. https://doi.org/10.3389/fbioe.2021.608826
Fengel D (1971) Ideas on the ultrastructural organization of the cell wall components. J Polym Sci Part C Polym Symp. https://doi.org/10.1002/polc.5070360127
Fengel D, Wegener G (2011) Wood: chemistry, ultrastructure, reactions. Walter de Gruyter, New York
Fernandes Diniz JMB, Gil MH, Castro JAAM (2004) Hornification—its origin and interpretation in wood pulps. Wood Sci Technol 37:489–494. https://doi.org/10.1007/s00226-003-0216-2
Ferreira JC, Evtuguin DV, Prates A (2020) Effect of cellulose structure on reactivity of eucalyptus acid sulphite dissolving pulp. Cellulose 27:4763–4772. https://doi.org/10.1007/s10570-020-03092-y
Fink HP, Ganster J, Lehmann A (2014) Progress in cellulose shaping: 20 years industrial case studies at Fraunhofer IAP. Cellulose 21:31–51. https://doi.org/10.1007/s10570-013-0137-7
Fink H-P, Weigel P, Purz HJ, Ganster J (2001) Structure formation of regenerated cellulose materials from NMMO-solutions. Prog Polym Sci 26:1473–1524. https://doi.org/10.1016/S0079-6700(01)00025-9
Fischer K, Schmidt I, Fischer S (2009) Reactivity of dissolving pulp for processing viscose. Macromol Symp 280:54–59. https://doi.org/10.1002/masy.200950607
Fischer S, Thümmler K, Volkert B, et al (2008) Properties and applications of cellulose acetate. In: Macromolecular symposia, pp 89–96
Fock W (1959) Eine modifizierte Methode zur Bestimmung der Reaktivität von Zellstoffen für die Viskoseherstellung. Das Papier 13:92–95
Friebel C, Bischof RH, Schild G et al (2019) Effects of caustic extraction on properties of viscose grade dissolving pulp. Processes 7:1–12. https://doi.org/10.3390/pr7030122
Froschauer C, Hummel M, Iakovlev M et al (2013) Separation of hemicellulose and cellulose from wood pulp by means of ionic liquid/cosolvent systems. Biomacromolecules 14:1741–1750
Fu J, Li X, Gao W et al (2012) Bio-processing of bamboo fibres for textile applications: a mini review. Biocatal Biotransformation 30:141–153. https://doi.org/10.3109/10242422.2012.650450
Gandini A (2011) The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem 13:1061–1083. https://doi.org/10.1039/c0gc00789g
García A, González Alriols M, Labidi J (2014) Evaluation of different lignocellulosic raw materials as potential alternative feedstocks in biorefinery processes. Ind Crops Prod 53:102–110. https://doi.org/10.1016/j.indcrop.2013.12.019
Gehmayr V, Schild G, Sixta H (2011) A precise study on the feasibility of enzyme treatments of a kraft pulp for viscose application. Cellulose 18:479–491. https://doi.org/10.1007/s10570-010-9483-x
Gehmayr V, Sixta H (2011) Dissolving pulp from enzyme treated kraft pulps. Lenzinger Berichte 89:152–160
Gehmayr V, Sixta H (2010) Feasibility of enzymatic bio-bleaching techniques of a kraft pulp for viscose application. In: International workshop on wood biorefinery and tree biotechnology. örnsköldsvik
Geng A, Xin F, Ip J (2012) Ethanol production from horticultural waste treated by a modified organosolv method. Bioresour Technol 104:715–721
Gondhalekar SC, Pawar PJ, Dhumal SS, Thakre SS (2019) Mechanism of xanthation reaction in viscose process. Cellulose 26:1595–1604. https://doi.org/10.1007/s10570-018-2213-5
Gray S (2017) Mapping clothing impacts in Europe: the environmental cost. WRAP. European Clothing Action Plan. Banbury (UK)
Grönqvist S, Hakala TK, Kamppuri T et al (2014) Fibre porosity development of dissolving pulp during mechanical and enzymatic processing. Cellulose 21:3667–3676
Grönqvist S, Kamppuri T, Maloney T et al (2015) Enhanced pre-treatment of cellulose pulp prior to dissolution into NaOH/ZnO. Cellulose 22:3981–3990. https://doi.org/10.1007/s10570-015-0742-8
Gullichsen J, Fogelholm C-J, McLeod M (1999) Book 6: Chemical pulping. The Finnish Paper Engineers’ Association, Helsinki
Gutiérrez A, Romero J, Del Rio JC (2001) Lipophilic extractives from Eucalyptus globulus pulp during kraft cooking followed by TCF and ECF bleaching. Holzforschung 55:260–264
Gübitz GMM, Lischnig T, Stebbing D, Saddler JNN (1997) Enzymatic removal of hemicellulose from dissolving pulps. Biotechnol Lett 19:491–495. https://doi.org/10.1023/A:1018364731600
Habibi Y (2014) Key advances in the chemical modification of nanocelluloses. Chem Soc Rev 43:1519–1542. https://doi.org/10.1039/c3cs60204d
Hammel KE (1997) Fungal degradation of lignin. Driven by nature: plant litter quality and decomposition, London, England
Haslinger S, Hummel M, Anghelescu-Hakala A et al (2019) Upcycling of cotton polyester blended textile waste to new man-made cellulose fibers. Waste Manage 97:88–96. https://doi.org/10.1016/j.wasman.2019.07.040
Haslinger S, Hummel M, Sixta H (2018) Separation and upcycling of cellulose-containing blended waste. Patent
Haule LV, Carr CM, Rigout M (2016) Preparation and physical properties of regenerated cellulose fibres from cotton waste garments. J Clean Prod 112:4445–4451. https://doi.org/10.1016/j.jclepro.2015.08.086
Hauru LKJ, Hummel M, King AWT et al (2012) Role of solvent parameters in the regeneration of cellulose from ionic liquid solutions. Biomacromol 13:2896–2905
Heinze T, El Seoud OA, Koschella A (2018) Cellulose derivatives: synthesis, structure, and properties. Springer series on polymer and composite materials. Springer, Cham, pp 531
Henriksson G, Christiernin M, Agnemo R (2005) Monocomponent endoglucanase treatment increases the reactivity of softwood sulphite dissolving pulp. J Ind Microbiol Biotechnol 32:211–214. https://doi.org/10.1007/s10295-005-0220-7
Hillman D (2006) Do dissolving pulps really dissolve. In: Paper Asia, pp 12–18
Hinck JF, Casebier RL, Hamilton JK (1985) Dissolving pulp manufacture. Pulp Pap Manuf 4:213–243
Hirota M, Tamura N, Saito T, Isogai A (2012) Cellulose II nanoelements prepared from fully mercerized, partially mercerized and regenerated celluloses by 4-acetamido-TEMPO/NaClO/NaClO2 oxidation. Cellulose 19:435–442
Hon DN-S (1994) Cellulose: a random walk along its historical path. Cellulose 1:1–25
Huang S, Tao R, Ismail A, Wang Y (2020) Cellulose nanocrystals derived from textile waste through acid hydrolysis and oxidation as reinforcing agent of soy protein film. Polymers 12:958. https://doi.org/10.3390/POLYM12040958
Hummel M, Laus G, Schwärzler A et al (2010) Non-halide ionic liquids for solvation, extraction, and processing of cellulosic materials. In: Liebert TF, Heinze TJ, Edgar KJ (eds) Cellulose solvents: for analysis, shaping and chemical modification. American Chemical Society, Washington, DC, pp 229–259
Hummel M, Michud A, Tanttu M et al (2015) Ionic liquids for the production of man-made cellulosic fibers: opportunities and challenges. Adv Polym Sci 271:133–168. https://doi.org/10.1007/12_2015_307
Hutterer C, Kliba G, Punz M et al (2017) Enzymatic pulp upgrade for producing high-value cellulose out of a Kraft paper pulp. Enzyme Microb Technol 102:67–73. https://doi.org/10.1016/j.enzmictec.2017.03.014
Hämmerle FM (2011) The cellulose gap (The future of cellulose fibres). Lenzinger Berichte 89:12–21
IONCELL (2023). https://ioncell.fi/commercialization/). Accessed: May 2023
Ibarra D, Köpcke V, Ek M (2010a) Behavior of different monocomponent endoglucanases on the accessibility and reactivity of dissolving-grade pulps for viscose process. Enzyme Microb Technol 47:355–362. https://doi.org/10.1016/j.enzmictec.2010.07.016
Ibarra D, Köpcke V, Larsson PT et al (2010b) Combination of alkaline and enzymatic treatments as a process for upgrading sisal paper-grade pulp to dissolving-grade pulp. Bioresour Technol 101:7416–7423. https://doi.org/10.1016/j.biortech.2010.04.050
Ingildeev D, Effenberger F, Bredereck K, Hermanutz F (2013) Comparison of direct solvents for regenerated cellulosic fibers via the lyocell process and by means of ionic liquids. J Appl Polym Sci 128:4141–4150. https://doi.org/10.1002/app.38470
Ishikawa A, Okano T, Sugiyama J (1997) Fine structure and tensile properties of ramie fibres in the crystalline form of cellulose I, II, IIII and IVI. Polymer 38:463–468. https://doi.org/10.1016/S0032-3861(96)00516-2
Jakes JE, Zelinka SL, Hunt CG et al (2020) Measurement of moisture-dependent ion diffusion constants in wood cell wall layers using time-lapse micro X-ray fluorescence microscopy. Sci Rep 10:9919. https://doi.org/10.1038/s41598-020-66916-8
Janzon R, Puls J, Bohn A et al (2008) Upgrading of paper grade pulps to dissolving pulps by nitren extraction: Yields, molecular and supramolecular structures of nitren extracted pulps. Cellulose 15:739–750. https://doi.org/10.1007/s10570-008-9224-6
Janzon R, Puls J, Saake B (2006) Upgrading of paper-grade pulps to dissolving pulps by nitren extraction: Optimisation of extraction parameters and application to different pulps. Holzforschung 60:347–354. https://doi.org/10.1515/HF.2006.055
Ji L, Zhao L (2015) Current situation and future trend of the dissolving market in domestic and international. China Pulp Pap Ind 36:41–45
Jiang X, Bai Y, Chen X, Liu W (2020) A review on raw materials, commercial production and properties of lyocell fiber. J Bioresour Bioprod 5:16–25
Kahoush M, Kadi N (2022) Towards sustainable textile sector: Fractionation and separation of cotton/ polyester fibers from blended textile waste. Sustain Mater Technol 34:e00513. https://doi.org/10.1016/j.susmat.2022.e00513
Kallio AMI (2021) Wood-based textile fibre market as part of the global forest-based bioeconomy. For Policy Econ 123:102364. https://doi.org/10.1016/j.forpol.2020.102364
Kamide K, Nishiyama K (2001) Cuprammonium processes. In: Woodings C (ed) Regenerated cellulose fibres. The Textile Institute, Cambridge, pp 88–155
Kaur P, Bhardwaj NK, Sharma J (2016) Pretreatment with xylanase and its significance in hemicellulose removal from mixed hardwood kraft pulp as a process step for viscose. Carbohydr Polym 145:95–102
Kerr J, Landry J (2017) Pulse of the fashion industry. Global Fashion Agenda & The Boston Consulting Group, Boston
Kihlman M, Wawro D, Perzon E, et al (2012) Preparation of cellulosic fibres in semi-pilot scale from NaOH-solutions. In: 243rd American Chemical Society (ACS) National Meeting. San Diego
King AWT, Zoia L, Filpponen I et al (2009) In situ determination of lignin phenolics and wood solubility in imidazolium chlorides using 31P NMR. J Agric Food Chem 57:8236–8243
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: Fascinating biopolymer and sustainable raw material. Angewandte Chemie - International Edition 44:3358–3393. https://doi.org/10.1002/anie.200460587
Klemm D, Philipp B, Heinze T et al (1998) Comprehensive cellulose chemistry. Volume I. Fundamentals and analytical methods. J Am Chem Soc 121:260. https://doi.org/10.1021/ja9857514
Kontturi E, Tammelin T, Österberg M (2006) Cellulose—model films and the fundamental approach. Chem Soc Rev 35:1287–1304. https://doi.org/10.1039/B601872F
Kosan B, Michels C, Meister F (2007) Dissolution and forming of cellulose with ionic liquids. Cellulose 15:59–66. https://doi.org/10.1007/s10570-007-9160-x
Krässig HA (1993) Cellulose: structure, accessibility and reactivity. Gordon and Breach Science Publication, Langhorne
Kumar H, Christopher LP (2017) Recent trends and developments in dissolving pulp production and application. Cellulose 24:2347–2365. https://doi.org/10.1007/s10570-017-1285-y
Kvarnlöf N, Germgård U, Jönsson LJ, Söderlund C-A (2006) Enzymatic treatment to increase the reactivity of a dissolving pulp for viscose preparation. Appita J 59:242–246
Köpcke V, Ibarra D, Ek M (2008) Increasing accessibility and reactivity of paper grade pulp by enzymatic treatment for use as dissolving pulp. Nord Pulp Paper Res J 23:363–368. https://doi.org/10.3183/npprj-2008-23-04-p363-368
Köpcke V (2010) Conversion of Wood and Non-wood Paper-grade Pulps to Dissolving-grade Pulps. Dissertation. KTH. Department of Fibre and Polymer Technology. Division of Wood Chemistry and Pulp Technology
LaNieve HL, Richard K (2007) Handbook of fiber chemistry. Cellulose acetate and triacetate fibers, 3rd edn. CRC Press, Boca Raton, pp 667–772
Larsson PT, Wickholm K, Iversen T (1997) A CP/MAS13C NMR investigation of molecular ordering in celluloses. Carbohydr Res 302:19–25. https://doi.org/10.1016/S0008-6215(97)00130-4
Latif W, Basit A, Rehman A et al (2019) Study of mechanical and comfort properties of modal with cotton and regenerated fibers blended woven fabrics. Jo Nat Fibers 16:836–845. https://doi.org/10.1080/15440478.2018.1441084
Laus G, Bentivoglio G, Schottenberger H et al (2005) Ionic liquids: current developments, potential and drawbacks for industrial applications. Lenzinger Berichte 84:71–85
Lawford HG, Rousseau JD (1993) Production of ethanol from pulp mill hardwood and softwood spent sulfite liquors by genetically engineered E. coli. Appl Biochem Biotechnol 39:667–685
Le Moigne N, Jardeby K, Navard P (2010) Structural changes and alkaline solubility of wood cellulose fibers after enzymatic peeling treatment. Carbohydr Polym 79:325–332. https://doi.org/10.1016/j.carbpol.2009.08.009
Li H, Legere S, He Z et al (2018) Methods to increase the reactivity of dissolving pulp in the viscose rayon production process: a review. Cellulose 25:3733–3753. https://doi.org/10.1007/s10570-018-1840-1
Li J, Liu Y, Duan C et al (2015a) Mechanical pretreatment improving hemicelluloses removal from cellulosic fibers during cold caustic extraction. Bioresour Technol 192:501–506
Li J, Zhang H, Duan C et al (2015b) Enhancing hemicelluloses removal from a softwood sulfite pulp. Bioresour Technol 192:11–16. https://doi.org/10.1016/j.biortech.2015.04.107
Lin N, Dufresne A (2014) Nanocellulose in biomedicine: current status and future prospect. Eur Polym J 59:302–325. https://doi.org/10.1016/J.EURPOLYMJ.2014.07.025
Liu Y, Shi L, Cheng D, He Z (2016) Dissolving pulp market and technologies: Chinese prospective—a mini-review. BioResources 11:7902–7916
Llaudet JM (1990) Modal blends in knitted and woven fabrics—fashion and functional aspects. Lenzinger Berichte 70:24–26
Loureiro PEG, Cadete SMS, Tokin R et al (2021) Enzymatic fibre modification during production of dissolving wood pulp for regenerated cellulosic materials. Front Plant Sci 12:1–9. https://doi.org/10.3389/fpls.2021.717776
Lv F, Yao D, Wang Y et al (2015) Recycling of waste nylon 6/spandex blended fabrics by melt processing. Compos B Eng 77:232–237. https://doi.org/10.1016/J.COMPOSITESB.2015.03.038
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. https://doi.org/10.1128/MMBR.66.3.506-577.2002
Ma X, Huang L, Chen Y et al (2011) Preparation of bamboo dissolving pulp for textile production. Part 1. Study on prehydrolysis of green bamboo for producing dissolving pulp. BioResources 6:1428–1439
Ma Y, Hummel M, Kontro I, Sixta H (2018) High performance man-made cellulosic fibres from recycled newsprint. Green Chem 20:160–169. https://doi.org/10.1039/c7gc02896b
Ma Y, Hummel M, Määttänen M et al (2016) Upcycling of waste paper and cardboard to textiles. Green Chem 18:858–866. https://doi.org/10.1039/c5gc01679g
Madsen B, Gamstedt EK (2013) Wood versus plant fibers: similarities and differences in composite applications. Adv Mater Sci Eng 2013:1–14. https://doi.org/10.1155/2013/564346
Magdzinski L (2006) Tembec temiscaming integrated biorefinery. Pulp Pap Can 107:44–46
Magina S, Barros-Timmons A, Ventura SPM, Evtuguin DV (2021) Evaluating the hazardous impact of ionic liquids—challenges and opportunities. J Hazard Mater 412:125215. https://doi.org/10.1016/j.jhazmat.2021.125215
Maharjan B, Park J, Kaliannagounder VK et al (2021) Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr Polym 251:117023. https://doi.org/10.1016/J.CARBPOL.2020.117023
Maloney TC, Paulapuro H (1999) The formation of pores in the cell wall. J Pulp Pap Sci 25:430–436
Maloney TC, Paulapuro H (2000) Effect of drying conditions on the swelling and bonding properties of bleached kraft hardwood pulp. In: 54th APPITA annual conference, pp 43–46
Marques G, Rencoret J, Gutiérrez A et al (2010) Evaluation of the chemical composition of different non-woody plant fibers used for pulp and paper manufacturing. Open Agric Journal 4:93–101
Mateos-Espejel E, Radiotis T, Jemaa N (2013) Implications of converting a kraft pulp mill to a dissolving pulp operation with a hemicellulose extraction stage. Tappi J 12:29–38
Mazza M, Catana D-A, Vaca-Garcia C, Cecutti C (2009) Influence of water on the dissolution of cellulose in selected ionic liquids. Cellulose 16:207–215
Mboowa D (2021) A review of the traditional pulping methods and the recent improvements in the pulping processes. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-01243-6
Mendes ISF, Prates A, Evtuguin DV (2021) Production of rayon fibres from cellulosic pulps: state of the art and current developments. Carbohydr Polym 273:118466. https://doi.org/10.1016/j.carbpol.2021.118466
Miao Q, Chen L, Huang L et al (2014) A process for enhancing the accessibility and reactivity of hardwood kraft-based dissolving pulp for viscose rayon production by cellulase treatment. Bioresour Technol 154:109–113. https://doi.org/10.1016/j.biortech.2013.12.040
Miao Q, Tian C, Chen L et al (2015) Combined mechanical and enzymatic treatments for improving the Fock reactivity of hardwood kraft-based dissolving pulp. Cellulose 22:803–809. https://doi.org/10.1007/s10570-014-0495-9
Michud A, King AWT, Parviainen AP, et al (2014) Process for the production of shaped cellulose articles from a solution containing pulp dissolved in distillable ionic liquids. Patent
Mitchell RL (1949) Viscose processing of cellulose. Ind Eng Chem 41:2197–2201
Moniruzzaman M, Goto M (2011) Ionic liquids: future solvents and reagents for pharmaceuticals. J Chem Eng Jpn 44:370–381. https://doi.org/10.1252/jcej.11we015
Moon RJ, Martini A, Nairn J et al (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 4:3941–3994. https://doi.org/10.1039/c0cs00108b
Mäki-Arvela P, Anugwom I, Virtanen P et al (2010) Dissolution of lignocellulosic materials and its constituents using ionic liquids—a review. Ind Crops Prod 32:175–201
Navone L, Moffitt K, Hansen K-A et al (2020) Closing the textile loop: enzymatic fibre separation and recycling of wool/polyester fabric blends. Waste Manag 102:149–160. https://doi.org/10.1016/j.wasman.2019.10.026
Nayeem J, Sarkar M, Quadery AH, Jahan MS (2017) High purity dissolving pulp from jute. Nordic Pulp Pap Res 32:623–629. https://doi.org/10.3183/NPPRJ-2017-32-04-p623-629
Nevell T, Zeronian S (1985) Formation of fibers from cellulose solutions. Cellulose Chemistry and its applications. Ellis Horwood Ltd., Chichester, England
Newman RH (2004) Carbon-13 NMR evidence for cocrystallization of cellulose as a mechanism for hornification of bleached kraft pulp. Cellulose 11:45–52. https://doi.org/10.1023/B:CELL.0000014768.28924.0c
Ngene GI, Roux J-C, Lachenal D (2022) Influence of Hollander Beater Refining on Xylan extraction from hardwood paper pulp by cold caustic extraction and xylanase treatment. Bioresources 17:908–921. https://doi.org/10.15376/biores.17.1.908-921
Nishiyama Y, Kim U-J, Kim D-Y et al (2003) Periodic disorder along ramie cellulose microfibrils. Biomacromol 4:1013–1017. https://doi.org/10.1021/bm025772x
OECD-FAO Agricultural Outlook. http://www.compareyourcountry.org/agricultural-outlook/en/2/CT/all/default/all/CT. Accessed: June 2023
Oksanen T, Buchert J, Viikari L (1997) The role of hemicelluloses in the hornification of bleached kraft pulps. Holzforschung 51:355–360. https://doi.org/10.1515/hfsg.1997.51.4.355
Olszewska AM (2013) Interfacial forces in nanocellulose based composite materials. Dissertation. Aalto University. Department of Forest Products Technology
Ouchi A, Toida T, Kumaresan S et al (2010) A new methodology to recycle polyester from fabric blends with cellulose. Cellulose 17:215–222. https://doi.org/10.1007/s10570-009-9358-1
O’Sullivan AC (1997) Cellulose: the structure slowly unravels. Cellulose 4:173–207. https://doi.org/10.1023/A:1018431705579
Palme A, Peterson A, de la Motte H et al (2017) Development of an efficient route for combined recycling of PET and cotton from mixed fabrics. Text Cloth Sustain 3:1–9. https://doi.org/10.1186/s40689-017-0026-9
Parviainen A, Wahlström R, Liimatainen U et al (2015) Sustainability of cellulose dissolution and regeneration in 1,5-diazabicyclo[4.3.0]non-5-enium acetate: a batch simulation of the IONCELL-F process. RSC Adv 5:69728–69737. https://doi.org/10.1039/c5ra12386k
Paulitz J, Sigmund I, Kosan B, Meister F (2017) Lyocell fibers for textile processing derived from organically grown hemp. Procedia Eng 200:260–268. https://doi.org/10.1016/j.proeng.2017.07.037
Paunonen S, Kamppuri T, Katajainen L et al (2019) Environmental impact of cellulose carbamate fibers from chemically recycled cotton. J Clean Prod 222:871–881. https://doi.org/10.1016/j.jclepro.2019.03.063
Penttilä PA, Várnai A, Leppänen K et al (2010) Changes in submicrometer structure of enzymatically hydrolyzed microcrystalline cellulose. Biomacromol 11:1111–1117
Pepper LR (2017) Organic cotton market report. http://textileexchange.org/downloads/2017-preferred-fiber-materials-market-report/
Philipp B (1993) Organic solvents for cellulose as a biodegradable polymer and their applicability for cellulose spinning and derivatization. J Macromol Sci Part A Pure Appl Chem 30:703–714. https://doi.org/10.1080/10601329308021256
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:6712–6728
Protz R, Weidel G, Lehmann A (2018) Man-made cellulosic fibers via the viscose process - new opportunities by cellulose carbamate. Lenzinger Berichte 94:77–84
Puls J, Janzon R, Saake B (2006) Comparative removal of hemicelluloses from paper pulps using nitren, cuen, NaOH, and KOH. Lenzinger Berichte 86:63–70
Pönni R, Rautkari L, Hill CAS, Vuorinen T (2014) Accessibility of hydroxyl groups in birch kraft pulps quantified by deuterium exchange in D2O vapor. Cellulose 21:1217–1226. https://doi.org/10.1007/s10570-014-0166-x
Pönni R (2014) Changes in accessibility of cellulose for kraft pulps measured by deuterium exchange. Dissertation. Aalto University. Department of Forest Products Technology
Quintana E, Ago M, Valls C et al (2018) Alternative chemo-enzymatic treatment for homogeneous and heterogeneous acetylation of wood fibers. Cellulose 25:5323–5336. https://doi.org/10.1007/s10570-018-1947-4
Quintana E, Valls C, Vidal T, Roncero MB (2015a) Comparative evaluation of the action of two different endoglucanases. Part II: on a biobleached acid sulphite pulp. Cellulose 22:2081–2093. https://doi.org/10.1007/s10570-015-0631-1
Quintana E, Valls C, Vidal T, Roncero MB (2015b) Comparative evaluation of the action of two different endoglucanases. Part I: on a fully bleached, commercial acid sulfite dissolving pulp. Cellulose 22:2067–2079. https://doi.org/10.1007/s10570-015-0623-1
Ramamoorthy SK, Skrifvars M, Persson A (2015) A review of natural fibers used in biocomposites: plant, animal and regenerated cellulose fibers. Polym Rev 55:107–162
Rebuzzi F, Evtuguin DV (2005) Effect of glucuronoxylan on the hornification of Eucalyptus globulus bleached pulps. Macromol Symp 232:121–128. https://doi.org/10.1002/masy.200551414
Risi (2013) Fortress paper to operate its specialty cellulose mill as swing mill to also produce NBHK pulp. https://www.pulpandpapercanada.com/thurso-returns-to-hardwood-kraft-pulp-1002656334/
Rodríguez A, Espinosa E, Domínguez-Robles J et al (2018) Different solvents for organosolv pulping. In: Kazi SN (ed) Pulp and paper processing. IntechOpen, Rijeka, p 2. https://doi.org/10.5772/intechopen.68843
Roeder T (2017) The viscose process: scope and limitations. FP1205 COST Stockholm: COST 7–9
Roselli A, Hummel M, Monshizadeh A et al (2014) Ionic liquid extraction method for upgrading eucalyptus kraft pulp to high purity dissolving pulp. Cellulose 21:3655–3666. https://doi.org/10.1007/s10570-014-0344-x
Roselli A, Froschauer C, Sixta H (2013) IONCELL: Selective xylan extraction with ionic liquids. In: The 17th international symposium on wood, fibre and pulping chemistry (ISWFPC), June 12–14, 2013, Vancouver, BC, p pp–xx
Rosenau T, Potthast A, Sixta H, Kosma P (2001) The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog Polym Sci 26:1763–1837. https://doi.org/10.1016/S0079-6700(01)00023-5
Rosson L, Byrne N (2020) Comparative gate-to-gate life cycle assessment for the alkali and acid pre-treatment step in the chemical recycling of waste cotton. Sustainability 12:1–15. https://doi.org/10.3390/su12208613
Saalwächter K, Burchard W, Klüfers P et al (2000) Cellulose solutions in water containing metal complexes. Macromolecules 33:4094–4107
Šajn N (2019) Environmental impact of the textile and clothing industry. What consumers need to know. Belgium
Santos AS, Ferreira PJT, Maloney T (2021) Bio-based materials for nonwovens. Cellulose 28:8939–8969. https://doi.org/10.1007/s10570-021-04125-w
Sappi (2020) Cloquet Mill. https://www.sappi.com/cloquet-mill
Sarkar M, Nayeem J, Popy RS et al (2018) Dissolving pulp from jute wastes. Bioresour Technol Rep 4:96–100. https://doi.org/10.1016/j.biteb.2018.09.008
Sayyed AJ, Deshmukh NA, Pinjari DV (2019) A critical review of manufacturing processes used in regenerated cellulosic fibres: viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 26:2913–2940. https://doi.org/10.1007/s10570-019-02318-y
Sayyed AJ, Mohite LV, Deshmukh NA, Pinjari DV (2020) Intensification of lyocell dissolution process and dope characteristics using pre-swelled cellulosic pulp. Chem Eng Process 148:107826. https://doi.org/10.1016/j.cep.2020.107826
Schaumann W (1996) Properties of Lenzing viscose and Lenzing modal applied to finishing. LenzingerBerichte75/96, pp81–90 http://www.lenzing.com/sites/fe/content/publikationen/lenzingerberichte/Dokumente/75_1996/LB-0751996081.pdf
Schild G, Sixta H (2011) Sulfur-free dissolving pulps and their application for viscose and lyocell. Cellulose 18:1113–1128. https://doi.org/10.1007/s10570-011-9532-0
Schild G, Sixta H, Testova L (2010) Multifunctional alkaline pulping, delignification and hemicellulose extraction. Cellul Chem Technol 44:35–45
Schultz T, Suresh A (2017) Life cycle assessment: comparing ten sources of manmade cellulose fiber. SCS Global Services, California
Sczostak A (2009) Cotton linters: an alternative cellulosic raw material. Macromol Symp 280:45–53. https://doi.org/10.1002/masy.200950606
Sears KD, Hinck JF, Sewell CG (1982) Highly reactive wood pulps for cellulose acetate production. J Appl Polym Sci 27:4599–4610
Shen L, Worrell E, Patel MK (2010) Environmental impact assessment of man-made cellulose fibres. Resour Conserv Recycl 55:260–274. https://doi.org/10.1016/j.resconrec.2010.10.001
Sinclair R (2015) Textiles and fashion: materials, design and technology. Woodhead Publishing, Cambridge
Sixta H (1998) Comparative evaluation of different concepts of sulfite pulping technology. Das Papier 52:239–249
Sixta H (2000) Comparative evaluation of TCF bleached hardwood dissolving pulps. Lenzinger Berichte 79:119–128
Sixta H (2008) Handbook of pulp. Wiley, New York
Sixta H, Iakovlev M, Testova L et al (2013) Novel concepts of dissolving pulp production. Cellulose 20:1547–1561. https://doi.org/10.1007/s10570-013-9943-1
Sixta H, Michud A, Hauru L et al (2015) Ioncell-F: a high-strength regenerated cellulose fibre. Nord Pulp Paper Res J 30:43–57. https://doi.org/10.3183/npprj-2015-30-01-p043-057
Sjahro N, Yunus R, Abdullah LC et al (2021) Recent advances in the application of cellulose derivatives for removal of contaminants from aquatic environments. Springer, Cham
Sjöström E (1993) Wood chemistry: fundamentals and applications, 2nd edn. Academic Press, San Diego
Sjöström E, Westermark U (1999) Chemical composition of wood and pulps: basic constituents and their distribution. In: Analytical methods in wood chemistry, pulping, and papermaking. Springer, Berlin, pp 1–19
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082
Sohn J, Nielsen KS, Birkved M et al (2021) The environmental impacts of clothing: evidence from United States and three European countries. Sustain Prod Consum 27:2153–2164. https://doi.org/10.1016/j.spc.2021.05.013
Statista. Global chemical fibers production 2000–2022, by type https://www.statista.com/global-production-of-the-chemical-fiber-industry). Accessed February 2023
Statista. Global production volume of textile fibers 1975–2022. https://www.statista.com/worldwideproduction. Accessed February 2023
Statista Worldwide production volume of chemical and textile fibers from 1975 to 2020. https://www.statista.com/statistics/263154/worldwide-production-volume-of-textile-fibers-since-1975/. Accessed 12 Jan 2022
Stepan AM, Michud A, Hellstén S et al (2016) IONCELL-P&F: pulp fractionation and fiber spinning with ionic liquids. Ind Eng Chem Res 55:8225–8233. https://doi.org/10.1021/acs.iecr.6b00071
Stone JE, Scallan AM (1968) The effect of component removal upon the porous structure of the cell wall of wood. Part III. A comparison between the sulphite and kraft processes. Pulp Pap Mag Can 6:69–74
Stone JE, Scallan AM, Donefer E, Ahlgren E (1969) Digestibility as a simple function of a molecule of similar size to a cellulase enzyme. In: Advances in chemistry. ACS Publications, pp 219–241
Stora Enso (2019) Stora Enso uniquely positioned in dissolving pulp. Stora Enso Newsroom
Strunk P, Eliasson B, Hagglund C, Agnemo R (2011) The influence of properties in cellulose pulps on the reactivity in viscose manufacturing. Nordic Pulp Pap Res 26:81–89. https://doi.org/10.3183/npprj-2011-26-01-p081-089
Strunk P, Lindgren Å, Agnemo R, Eliasson B (2012a) Properties of cellulose pulps and their influence on the production of a cellulose ether. Nord Pulp Paper Res J 27:24–34. https://doi.org/10.3183/npprj-2012-27-01-p024-034
Strunk P, Lindgren Å, Eliasson B, Agnemo R (2012b) Chemical changes of cellulose pulps in the processing to viscose dope. Cellul Chem Technol 46:559–569
Sun N, Rahman M, Qin Y et al (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem 11:646–655
Suurnäkki A, Li T-Q, Buchert J et al (1997) Effects of enzymatic removal of xylan and glucomannan on the pore size distribution of kraft fibres. Holzforschung 51:27–33
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc 124:4974–4975. https://doi.org/10.1021/ja025790m
Syed HU, Nebamoh IP, Germgard U (2013) A comparison of cold and hot caustic extraction of a spruce dissolving sulfite pulp prior to final bleaching. Appita Technol Innov Manuf Environ 66:229–234
TENCEL (2023). https://www.tencel.com/b2b/product/tencel-modal. Accessed: May 2023
Teng Y, Yu G, Fu Y, Yin C (2018) The preparation and study of regenerated cellulose fibers by cellulose carbamate pathway. Int J Biol Macromol 107:383–392. https://doi.org/10.1016/j.ijbiomac.2017.09.006
Testova L, Borrega M, Tolonen LKLK et al (2014) Dissolving-grade birch pulps produced under various prehydrolysis intensities: quality, structure and applications. Cellulose 21:2007–2021. https://doi.org/10.1007/s10570-014-0182-x
Tian C, Zheng L, Miao Q et al (2014) Improving the reactivity of kraft-based dissolving pulp for viscose rayon production by mechanical treatments. Cellulose 21:3647–3654. https://doi.org/10.1007/s10570-014-0332-1
Treiber E, Rehnström J, Ameen C, Kolos F (1962) Using a laboratory viscose small-scale plant to test chemical conversion pulps. Das Papier 16:85–94
UNECE/FAO (2019) Forests for Fashion - For Sustainable Development Goals. https://unece.org/DAM/timber/Communications/Forests_for_Fashion_Booklet_2019.pdf
Vallejos ME, Olmos GV, Taleb MC et al (2022) Dissolving pulp from eucalyptus sawdust for regenerated cellulose products. Cellulose 29:4645–4659. https://doi.org/10.1007/s10570-022-04581-y
Van Woensel L, Lipp S (2020) Scientific Foresight: What if fashion were good for the planet? European Parliamentary Research Service https://www.europarl.europa.eu/RegData/etudes/ATAG/2020/656296/EPRS_ATA(2020)656296_EN.pdf
Vanzetto AB, Beltrami LVR, Zattera AJ (2021) Textile waste as precursors in nanocrystalline cellulose synthesis. Cellulose 28:6967–6981. https://doi.org/10.1007/s10570-021-03982-9
Vehviläinen M, Kamppuri T, Rom M et al (2008) Effect of wet spinning parameters on the properties of novel cellulosic fibres. Cellulose 15:671–680. https://doi.org/10.1007/s10570-008-9219-3
Vigliani EC (1954) Carbon disulphide poisoning in viscose rayon factories. Br J Ind Med 11:235–244. https://doi.org/10.1136/oem.11.4.235
Vila C, Santos V, Parajó JC (2004) Dissolving pulp from TCF bleached Acetosolv beech pulp. J Chem Technol Biotechnol 79:1098–1104. https://doi.org/10.1002/jctb.1090
Viëtor RJ, Newman RH, Ha M et al (2002) Conformational features of crystal-surface cellulose from higher plants. Plant J 30:721–731. https://doi.org/10.1046/j.1365-313X.2002.01327.x
Wada M, Chanzy H, Nishiyama Y, Langan P (2004) Cellulose IIII crystal structure and hydrogen bonding by synchrotron x-ray and neutron fiber diffraction. Macromolecules 37:8548–8555. https://doi.org/10.1021/ma0485585
Wada M, Ike M, Tokuyasu K (2010) Enzymatic hydrolysis of cellulose I is greatly accelerated via its conversion to the cellulose II hydrate form. Polym Degrad Stab 95:543–548. https://doi.org/10.1016/j.polymdegradstab.2009.12.014
Wallis AFA, Wearne RH (1990) Chemical cellulose from radiata pine kraft pulp. Appita J
Wan C, Jiao Y, Wei S et al (2019) Functional nanocomposites from sustainable regenerated cellulose aerogels: a review. Chem Eng J 359:459–475. https://doi.org/10.1016/j.cej.2018.11.115
Wang H, Gurau G, Rogers RD (2012) Ionic liquid processing of cellulose. Chem Soc Rev 41:1519–1537. https://doi.org/10.1039/c2cs15311d
Wang S, Lu A, Zhang L (2016) Recent advances in regenerated cellulose materials. Prog Polym Sci 53:169–206. https://doi.org/10.1016/j.progpolymsci.2015.07.003
Wang H, Pang B, Wu K et al (2014) Two stages of treatments for upgrading bleached softwood paper grade pulp to dissolving pulp for viscose production. Biochem Eng J 82:183–187. https://doi.org/10.1016/j.bej.2013.11.019
Wang L, Zhang Y, Gao P et al (2006) Changes in the structural properties and rate of hydrolysis of cotton fibers during extended enzymatic hydrolysis. Biotechnol Bioeng 93:443–456
Wawro D, Hummel M, Michud A, Sixta H (2014) Strong cellulosic film cast from ionic liquid solutions. Fibres Text East Europe 3:35–42
Weißl M, Hobisch MA, Johansson LS et al (2019) Cellulose carbamate derived cellulose thin films: preparation, characterization and blending with cellulose xanthate. Cellulose 26:7399–7410. https://doi.org/10.1007/s10570-019-02600-z
Wong KKY, Deverell KF, Mackie KL et al (1988) The relationship between fiber-porosity and cellulose digestibility in steam-exploded Pinus radiata. Biotechnol Bioeng 31:447–456
Woodings CR (1995) The development of advanced cellulosic fibres. Int J Biol Macromol 17:305–309. https://doi.org/10.1016/0141-8130(96)81836-8
Woodings C (2001) Regenerated cellulose fibres. The Textile Institute, Cambridge
Wu C-J, Zhang J-C, Yu D-M, Li R-G (2017) Dissolving pulp from bamboo-willow. Cellulose 25:777–785. https://doi.org/10.1007/s10570-017-1596-z
Wu C, Zhou S, Zhao C, Wang D (2014) Improved reactivity of bamboo dissolving pulp for the viscose process: Post-treatment with beating. BioResources 9:3449–3455
Xia G, Han W, Xu Z et al (2021) Complete recycling and valorization of waste textiles for value-added transparent films via an ionic liquid. J Environ Chem Eng 9:106182. https://doi.org/10.1016/j.jece.2021.106182
Xu A, Zhang Y, Zhao Y, Wang J (2013) Cellulose dissolution at ambient temperature: role of preferential solvation of cations of ionic liquids by a cosolvent. Carbohydr Polym 92:540–544
Yang B, Qin X, Duan C et al (2021a) Converting bleached hardwood kraft pulp to dissolving pulp by using organic electrolyte solutions. Cellulose 28:1311–1320. https://doi.org/10.1007/s10570-020-03642-4
Yang L, Wu Y, Yang F et al (2021b) A wood textile fiber made from natural wood. J Mater Sci 56:15122–15133. https://doi.org/10.1007/s10853-021-06240-2
Yang S, Yang B, Duan C et al (2019) Applications of enzymatic technologies to the production of high-quality dissolving pulp: a review. Bioresour Technol 281:440–448. https://doi.org/10.1016/j.biortech.2019.02.132
Yousef S, Tatariants M, Tichonovas M et al (2020) Sustainable green technology for recovery of cotton fibers and polyester from textile waste. J Clean Prod 254:120078. https://doi.org/10.1016/j.jclepro.2020.120078
Zakrzewska ME, Bogel-Łukasik E, Bogel-Łukasik R (2010) Solubility of carbohydrates in ionic liquids. Energy Fuels 24:737–745
Zeng B, Wang X, Byrne N (2019) Development of cellulose based aerogel utilizing waste denim—a morphology study. Carbohydr Polym 205:1–7. https://doi.org/10.1016/j.carbpol.2018.09.070
Zhang S, Chen C, Duan C et al (2018) Regenerated cellulose by the lyocell process, a brief review of the process and properties. BioResources 13:4577–4592
Zhang J, Kitayama H, Gotoh Y et al (2019) Non-woven fabrics of fine regenerated cellulose fibers prepared from ionic-liquid solution via wet type solution blow spinning. Carbohydr Polym 226:115258. https://doi.org/10.1016/J.CARBPOL.2019.115258
Zhang JC, Cao L, Zhou YK (2006) Discussion on the process and performance research of hemp viscose fiber. In: Advanced materials research. pp 529–534
Zhao L, Yuan Z, Kapu NS et al (2017) Increasing efficiency of enzymatic hemicellulose removal from bamboo for production of high-grade dissolving pulp. Bioresour Technol 223:40–46
Zhou S, Li Y, Huang L et al (2018) Enhanced reactivity of kraft-based viscose-grade dissolving pulp by hollander beating treatment. Bioresources 13:2861–2870. https://doi.org/10.15376/biores.13.2.2861-2870
Zhou C, Wang Y (2021) Recycling of waste cotton fabrics into regenerated cellulose films through three solvent systems: a comparison study. J Appl Polym Sci 138:51255. https://doi.org/10.1002/app.51255
Zhu JY, Wang GS, Pan XJ, Gleisner R (2009) Specific surface to evaluate the efficiencies of milling and pretreatment of wood for enzymatic saccharification. Chem Eng Sci 64:474–485
Acknowledgements
This publication is part of Project PID2020-114070RB-I00 (CELLECOPROD), funded by MCIN / AEI / 10.13039 / 501100011033. The author Elisabet Quintana is a Serra Húnter Fellow.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Blanca Roncero conceived the article. Elisabet Quintana conducted the literature search, analyzed data and wrote the manuscript. Blanca Roncero and Cristina Valls critically revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quintana, E., Valls, C. & Roncero, M.B. Dissolving-grade pulp: a sustainable source for fiber production. Wood Sci Technol 58, 23–85 (2024). https://doi.org/10.1007/s00226-023-01519-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-023-01519-w