Abstract
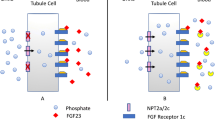
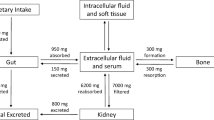
X-linked hypophosphatemic rickets (XLH) is a genetic cause of renal hypophosphatemia due to inactivation of the PHEX gene, with an inappropriate concentration of fibroblast growth factor 23 (FGF23). Burosumab, an anti-FGF23 monoclonal antibody, is a validated treatment for XLH, but its use in patients with chronic kidney disease (CKD) has not been validated. A 61-year-old man with XLH developed CKD during follow-up. Conventional treatment (phosphate salts and active vitamin D analogs) was poorly tolerated. Treatment with burosumab was decided at a multi-professional meeting. Before burosumab initiation, his measured glomerular filtration rate was 44 mL/min/1.73 m2 defining CKD stage 3b and intact FGF23 concentration was very high (4496.0 ng/mL, N: 22.7–93.1) due to both XLH and CKD. Severe hypophosphatemia was observed after the two first injections of burosumab at usual doses (1 mg/kg monthly) and concomitant discontinuation of the conventional treatment. After increasing the dose and reducing the interval between doses (1.3 mg/kg every three weeks) from the third injection, serum phosphate concentration normalized and remained around the lower limit of the normal range. A local cutaneous reaction was observed just after the second injection, but did not recur. We report for the first time the efficacy and good short-term tolerance of burosumab in a patient with XLH and CKD, subject to a higher dosage aimed at achieving a phosphatemia at the lower limit of the normal range.


Similar content being viewed by others
Data Availability
Data supporting the findings of this study will be made available upon reasonable request.
References
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res Off J Am Soc Bone Miner Res 19:429–435. https://doi.org/10.1359/JBMR.0301264
Rowe PSN (2012) Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr 22:61–86. https://doi.org/10.1615/critreveukargeneexpr.v22.i1.50
Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL (2011) A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res Off J Am Soc Bone Miner Res 26:1381–1388. https://doi.org/10.1002/jbmr.340
Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D, Wicart P et al (2019) Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol 15(7):435–455. https://doi.org/10.1038/s41581-019-0152-5
Imel EA, Glorieux FH, Whyte MP, Munns CF, Ward LM, Nilsson O, Simmons JH, Padidela R, Namba N, Cheong HI, Pitukcheewanont P, Sochett E, Högler W, Muroya K, Tanaka H, Gottesman GS, Biggin A, Perwad F, Mao M, Chen C-Y, Skrinar A, San Martin J, Portale AA (2019) Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet Lond Engl 393:2416–2427. https://doi.org/10.1016/S0140-6736(19)30654-3
Insogna KL, Briot K, Imel EA, Kamenický P, Ruppe MD, Portale AA, Weber T, Pitukcheewanont P, Cheong HI, Jan de Beur S, Imanishi Y, Ito N, Lachmann RH, Tanaka H, Perwad F, Zhang L, Chen C-Y, Theodore-Oklota C, Mealiffe M, San Martin J, Carpenter TO, AXLES 1 Investigators (2018) A Randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with x-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res Off J Am Soc Bone Miner Res 33:1383–1393. https://doi.org/10.1002/jbmr.3475
Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai M-M, Cattley RC, Wronski TJ, Xia X, Li X, Henley C, Eschenberg M, Richards WG (2012) FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 122:2543–2553. https://doi.org/10.1172/JCI61405
Courbebaisse M, Lanske B (2018) Biology of fibroblast growth factor 23: from physiology to pathology. Cold Spring Harb Perspect Med 8:a031260. https://doi.org/10.1101/cshperspect.a031260
Mäkitie O, Kooh SW, Sochett E (2003) Prolonged high-dose phosphate treatment: a risk factor for tertiary hyperparathyroidism in X-linked hypophosphatemic rickets. Clin Endocrinol (Oxf) 58:163–168. https://doi.org/10.1046/j.1365-2265.2003.01685.x
Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D, Wicart P, Bockenhauer D, Santos F, Levtchenko E, Harvengt P, Kirchhoff M, Di Rocco F, Chaussain C, Brandi ML, Savendahl L, Briot K, Kamenicky P, Rejnmark L, Linglart A (2019) Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol 15:435–455. https://doi.org/10.1038/s41581-019-0152-5
Giralt M, Chocron S, Ferrer R, Ariceta G (2021) Plasma intact fibroblast growth factor 23 level is a useful tool for diagnostic approach of renal hypophosphatemia. Pediatr Nephrol Berl Ger 36:1025–1028. https://doi.org/10.1007/s00467-020-04906-8
Piketty M-L, Brabant S, Souberbielle J-C, Maruani G, Audrain C, Rothenbuhler A, Prié D, Linglart A (2020) FGF23 measurement in burosumab-treated patients: an emerging treatment may induce a new analytical interference. Clin Chem Lab Med 58:e267–e269. https://doi.org/10.1515/cclm-2020-0460
Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121:4393–4408. https://doi.org/10.1172/JCI46122
Castellano-Martinez A, Acuñas-Soto S, Roldan-Cano V, Rodriguez-Gonzalez M (2022) Left ventricular hypertrophy in patients with X-linked hypophosphataemia. J Clin Res Pediatr Endocrinol 14:344–349. https://doi.org/10.4274/jcrpe.galenos.2021.2020.0287
Acknowledgements
The authors would like to thank Professor Pascal Houillier for his proofreading of this case report.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KB: fees for conference organization, board member of the XLH registry sponsored by KYOWA KIRIN PHARMA and participation in burosumab clinical trials conducted by KYOWA KIRIN PHARMA. GM: fees for moderating a round table during a conference organized by KYOWA KIRIN PHARMA. AMC, EB, DP, JN, and MC have no competing interests to declare that are relevant to the content of this article.
Human and Animal Rights and Informed Consent
The patient has been informed and has not objected to the use of this data for this publication, which has been collected in compliance with the requirements of the CNIL (Commission Nationale de l'Informatique et des Libertés). Under these conditions, recourse to an ethics commission is optional under French legislation. Signed consent to participate in research was obtained from our Physiology Department.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Michon-Colin, A., Bouderlique, E., Prié, D. et al. Successful Burosumab Treatment in an Adult Patient with X-Linked Hypophosphatemia and Chronic Kidney Disease Stage 3b. Calcif Tissue Int 114, 310–314 (2024). https://doi.org/10.1007/s00223-023-01169-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01169-x